1. The document discusses the electrical properties of cell membranes, including diffusion potential, equilibrium potential, and the Donnan effect.
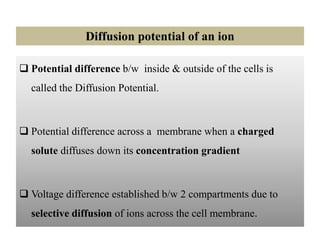
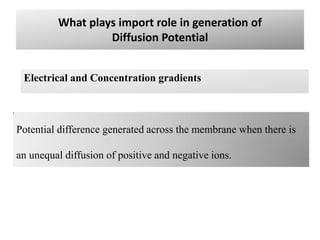
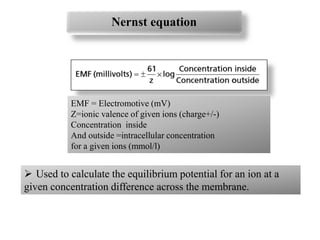
2. Diffusion potential is the voltage difference established across a membrane when ions diffuse down their concentration gradient through selective permeable pathways. The Nernst equation is used to calculate equilibrium potential for a single ion based on its intracellular and extracellular concentrations.
3. The Goldman equation calculates membrane potential based on permeability and concentrations of multiple permeable ions.
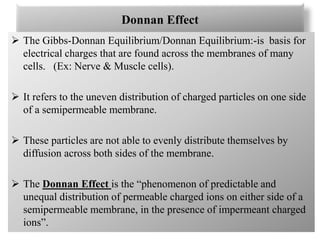
4. The Donnan effect refers to the uneven distribution of charged particles across a semipermeable membrane containing impermeable ions, maintaining concentration gradients and establishing a membrane potential.



![In Nerve fiber membrane:- [K+] > inside & < outside
Assume:- Membrane permeable to K+ ions only.
Strong tendency for extra K+ ions leads to its diffusion
outward.
Carry +ve electrical charges to the outside, thus
electropositivity outside the membrane & electronegativity
inside as negative anions remain behind.
Within millisecond diffusion potential becomes great enough to
block further net K+ ions diffusion to the exterior, despite the
high K+ ion concentration gradient.
Normal N fiber, the potential difference = -94 mV, [ -ve inside]](https://image.slidesharecdn.com/letcture39electricalpotential-i-201022203433/85/Letcture-39-electrical-potential-i-6-320.jpg)




![Ex: If the intracellular [Ca2+] is 10−7 mol/L and the extracellular
[Ca2+] is 2 × 10−3 mol/L, at what potential difference across the
cell membrane will Ca2+ be at electrochemical
equilibrium? Assume that 2.3RT/F = 60 mV at body temperature
(37°C).
SOLUTION. Given this concentration gradient across the
membrane, if Ca2+ is the only permeant ion. Remember, Ca2+ is
divalent, so z = +2.
Thus,](https://image.slidesharecdn.com/letcture39electricalpotential-i-201022203433/85/Letcture-39-electrical-potential-i-12-320.jpg)





![At equilibrium two things happen:-
I. The diffusible [K+] ions on the side of the membrane
containing the non-penetrating anion X- is greater than the
diffusible cation concentration on the other side
II. The diffusible anions [cl-] ions will be greater on the side
without the non-diffusible anion.
This is known as the Gibbs –Donnan membrane equilibrium](https://image.slidesharecdn.com/letcture39electricalpotential-i-201022203433/85/Letcture-39-electrical-potential-i-19-320.jpg)
