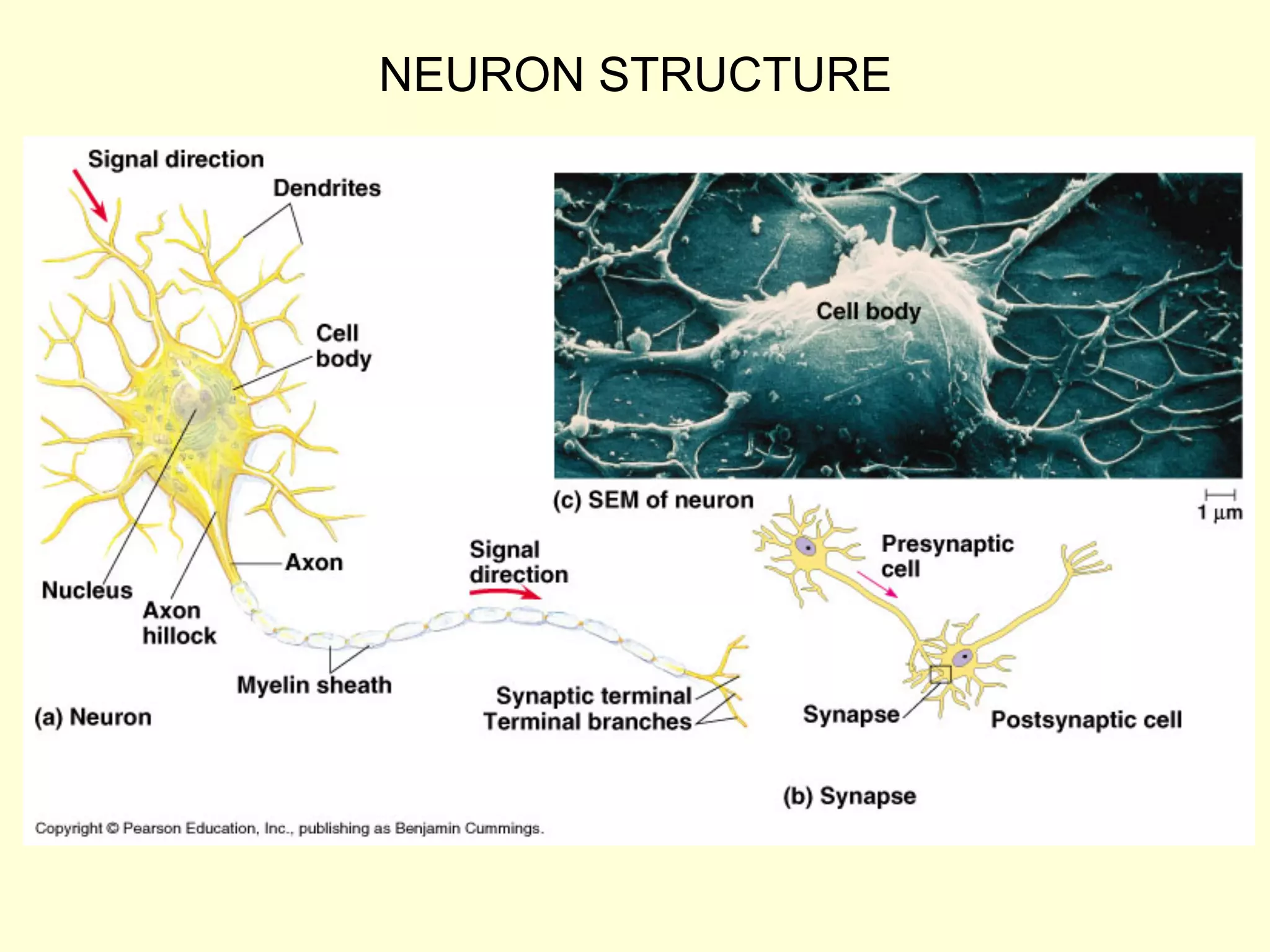
This document discusses several concepts related to the transport of ions across membranes:
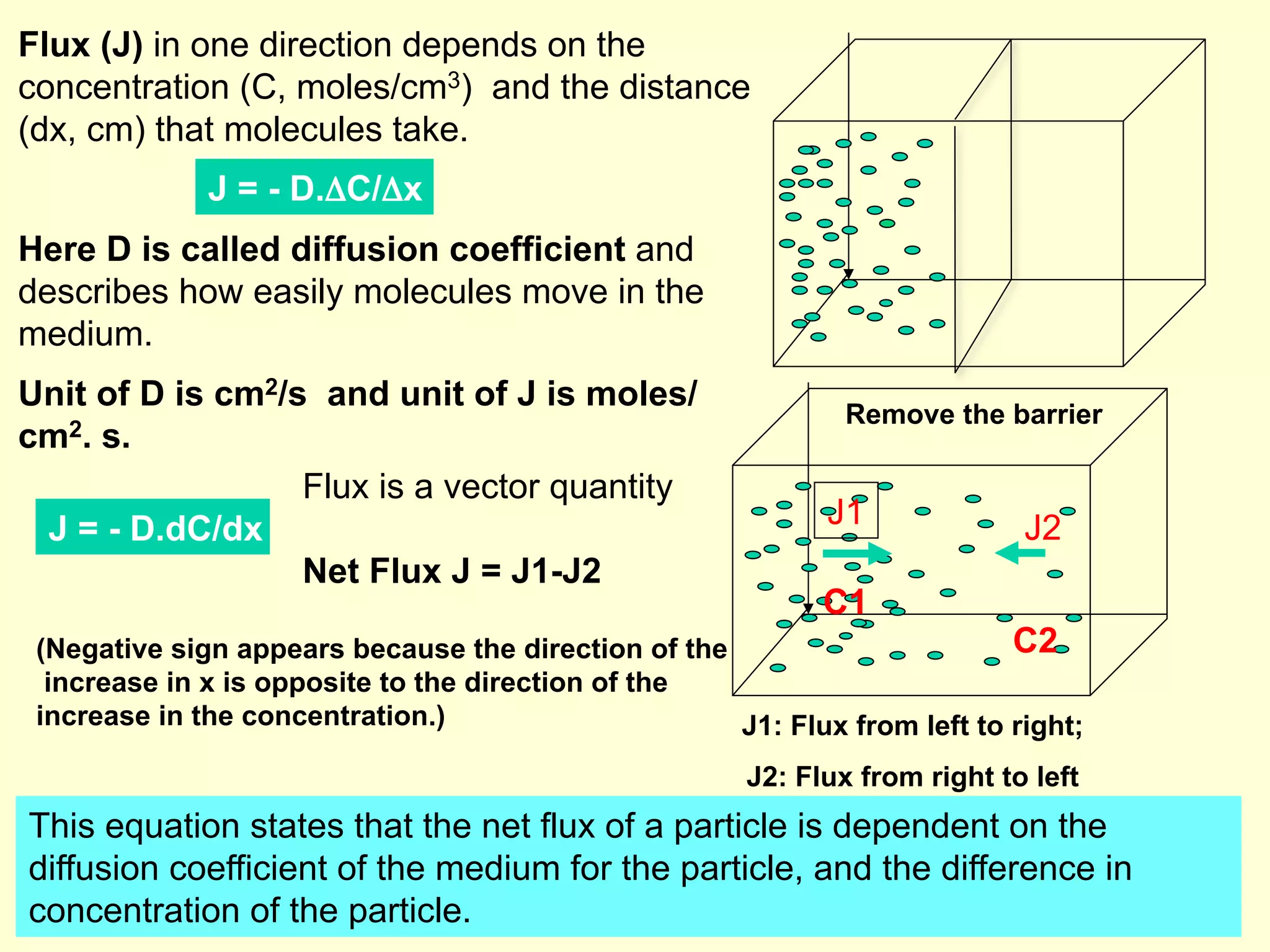
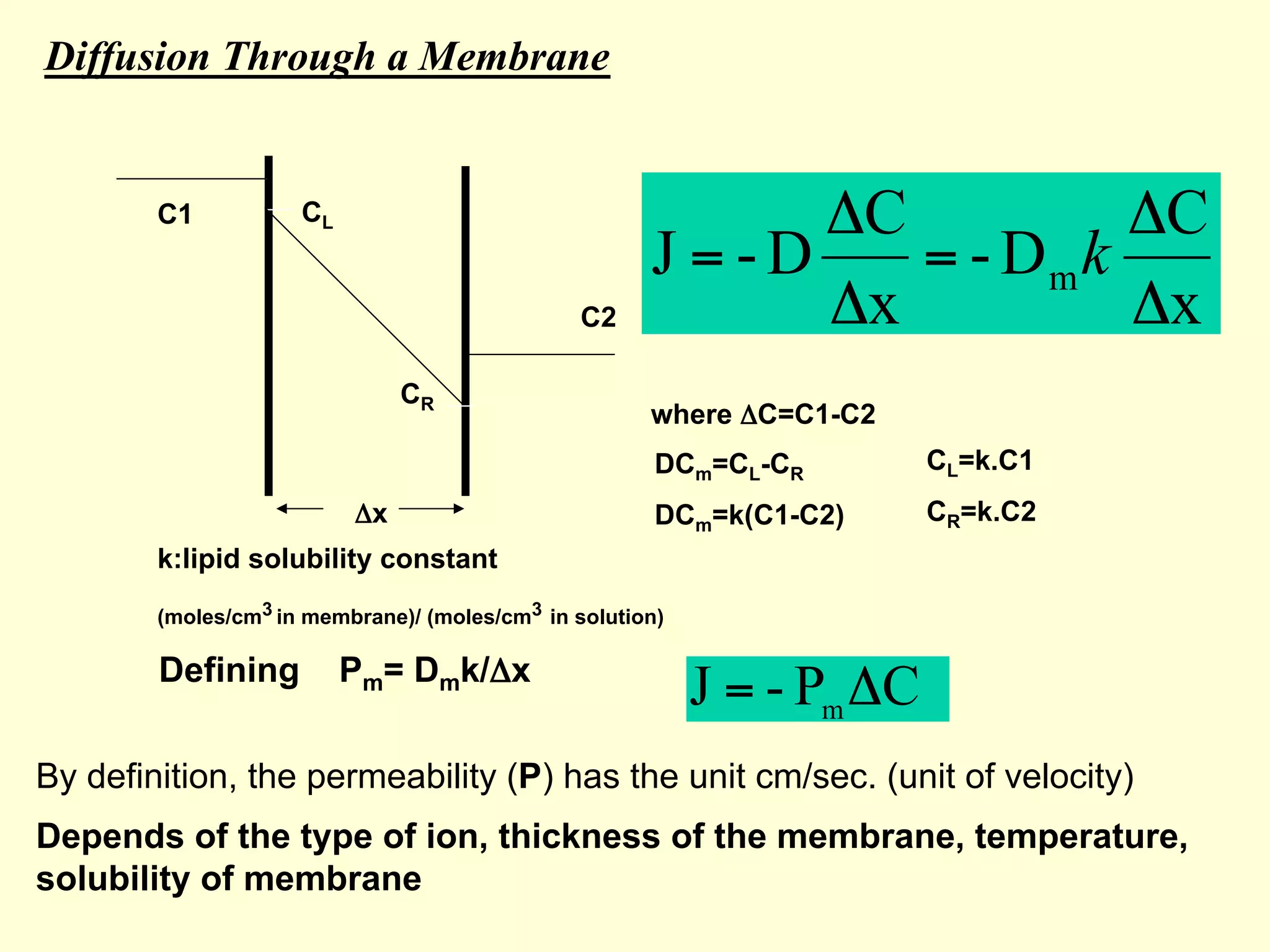
1) Ion transport is driven by concentration gradients and diffusion according to Fick's Law of diffusion. The flux of ions is proportional to the concentration gradient and the permeability of the membrane.
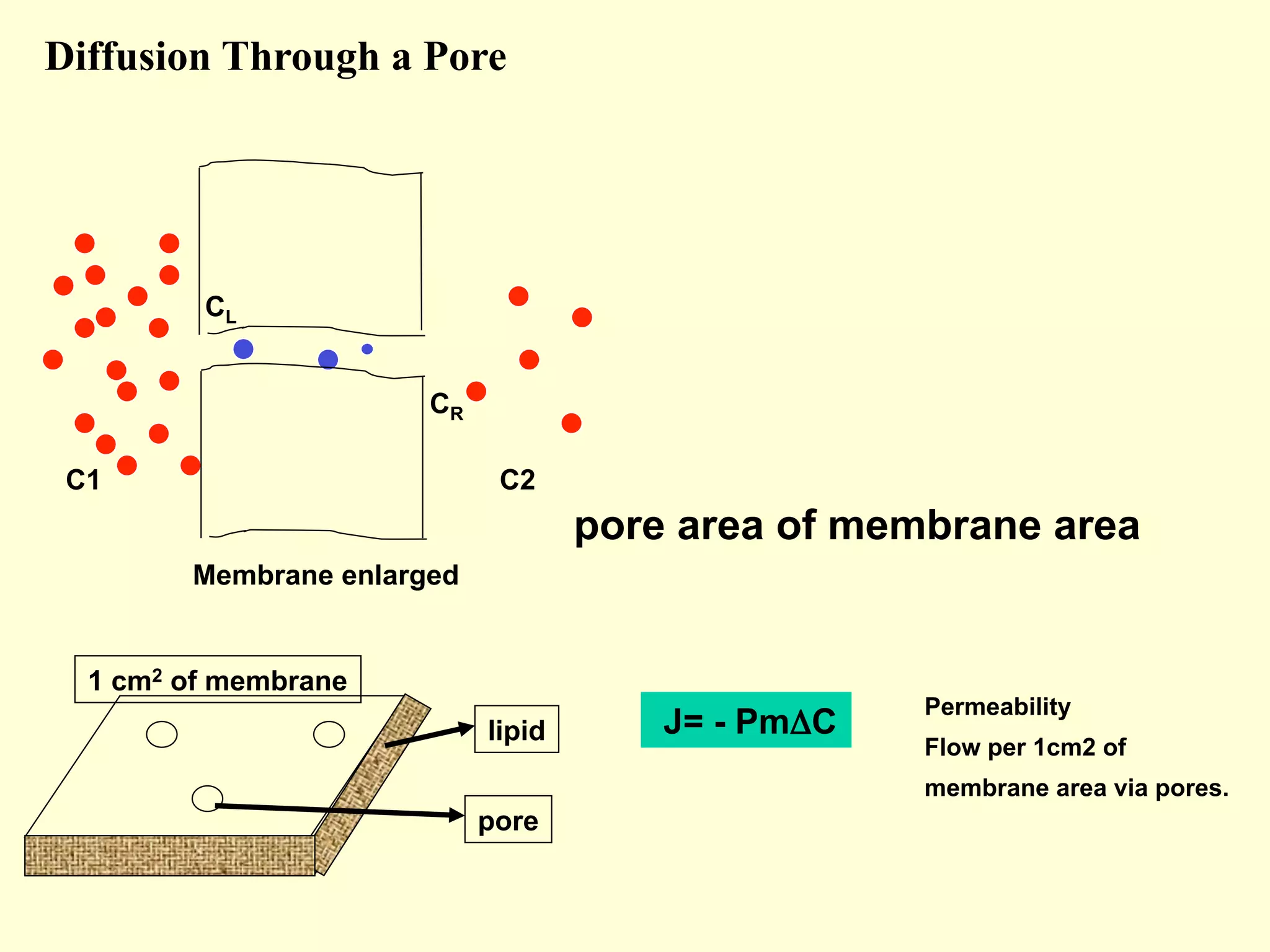
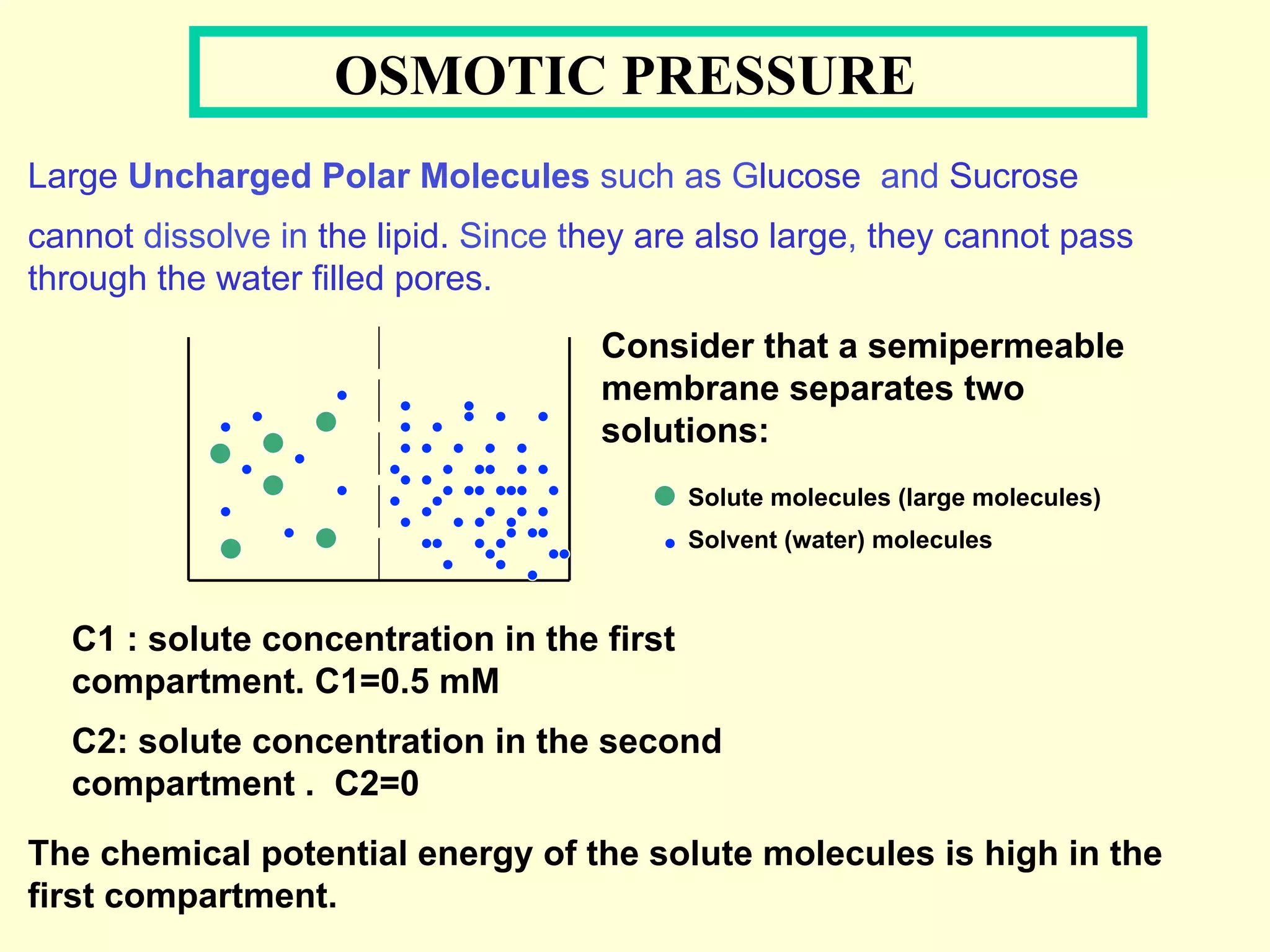
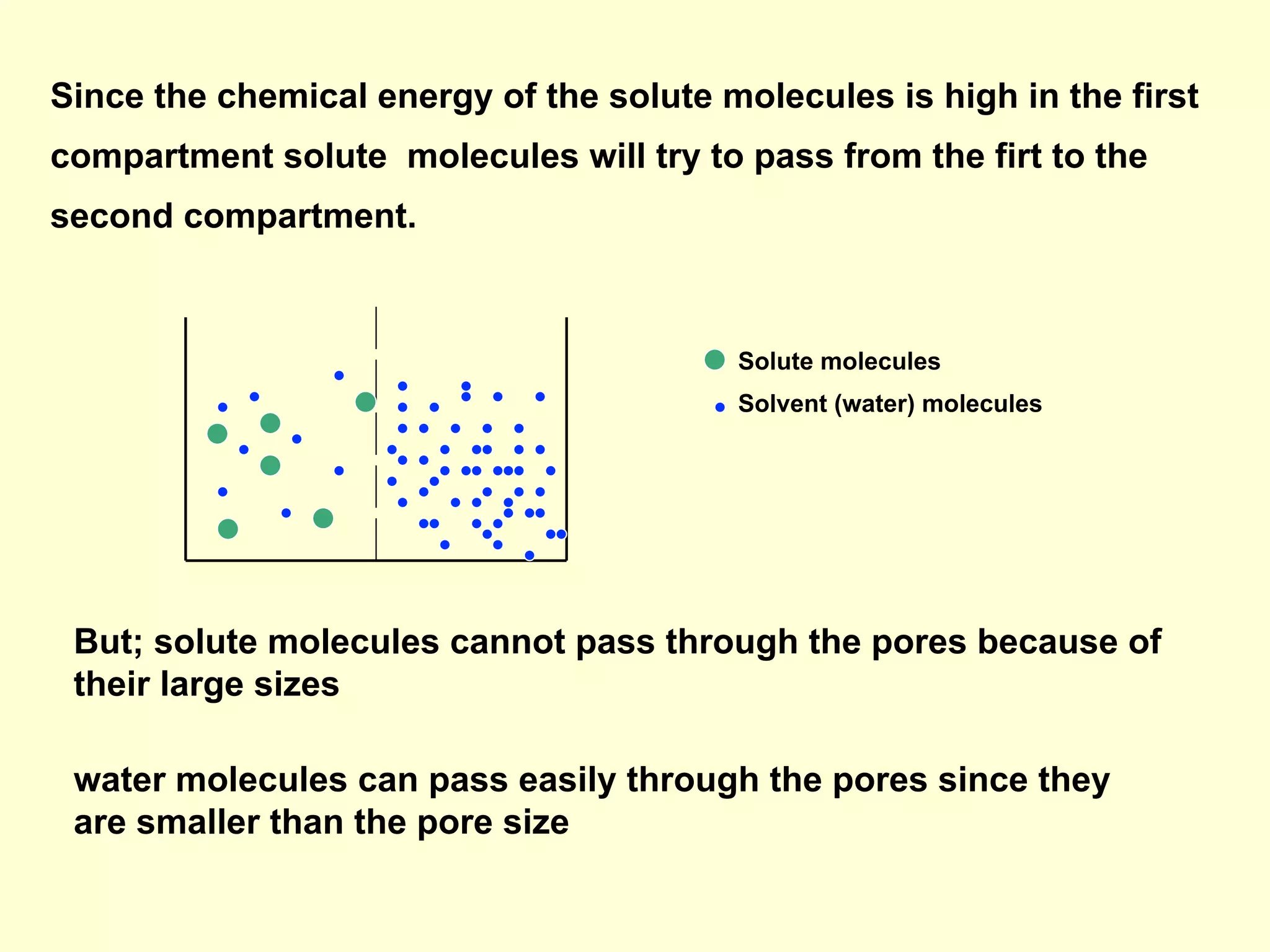
2) Membranes are selectively permeable, allowing some ions to pass freely through aqueous pores while blocking other larger ions and molecules.
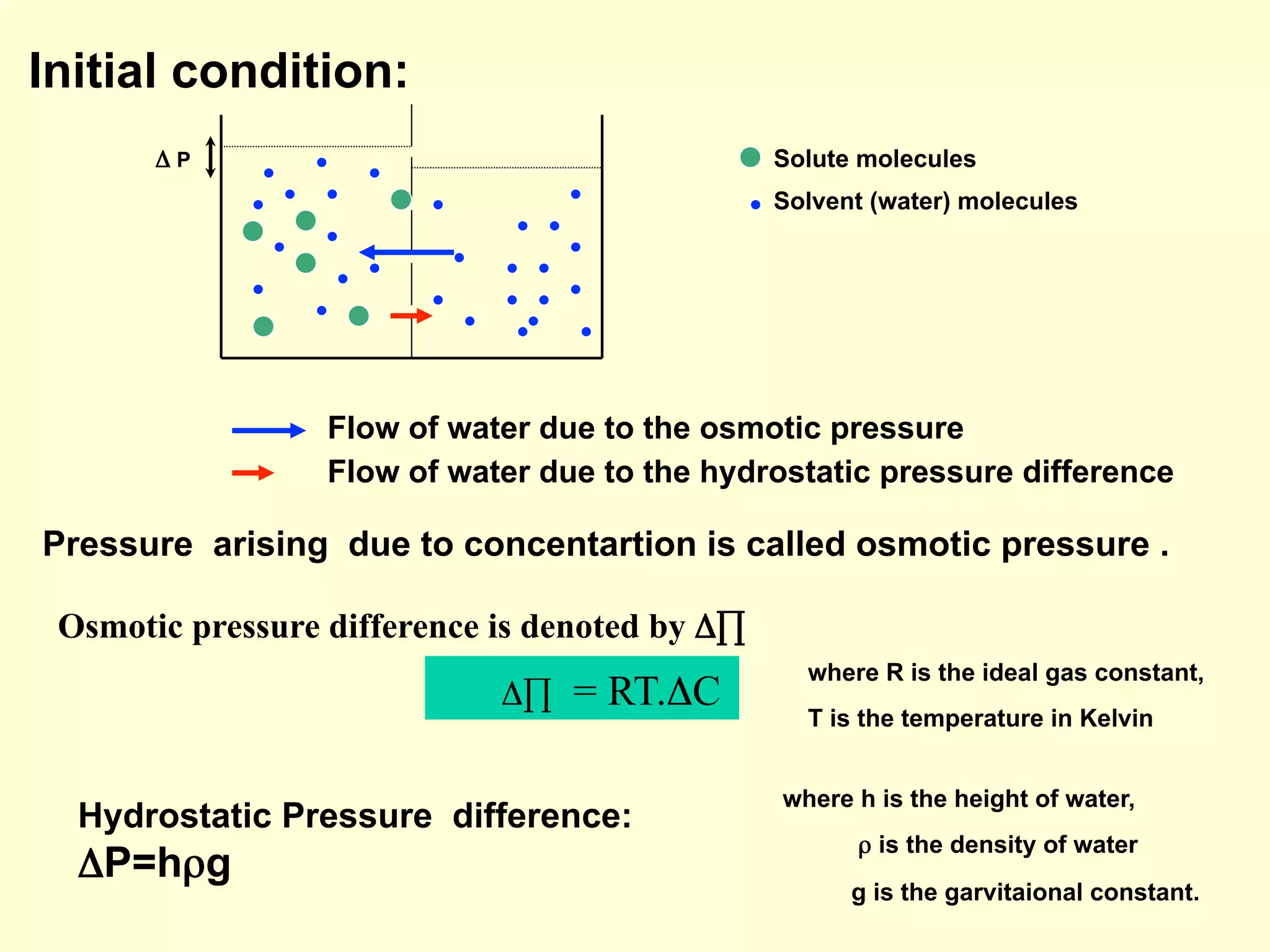
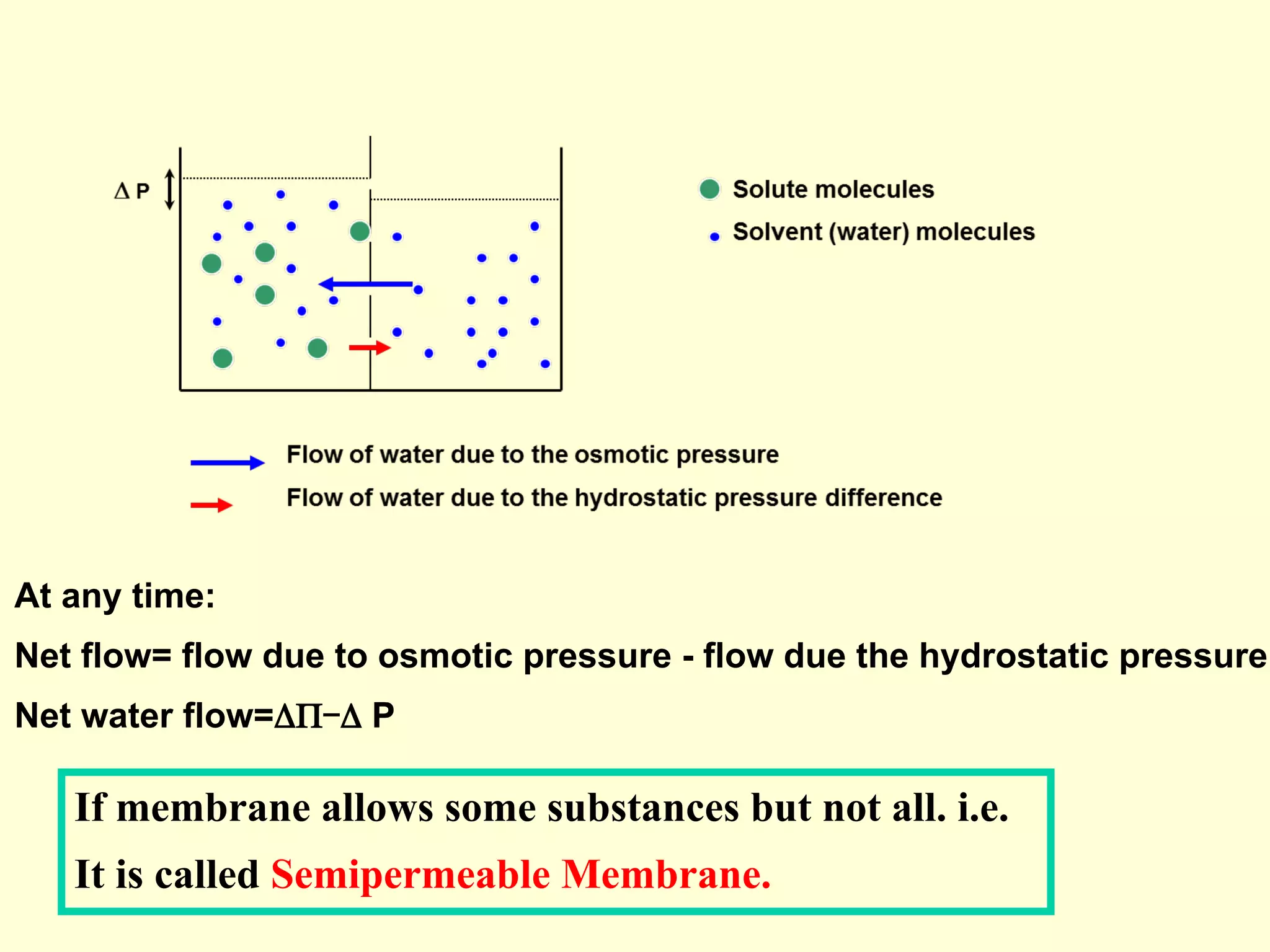
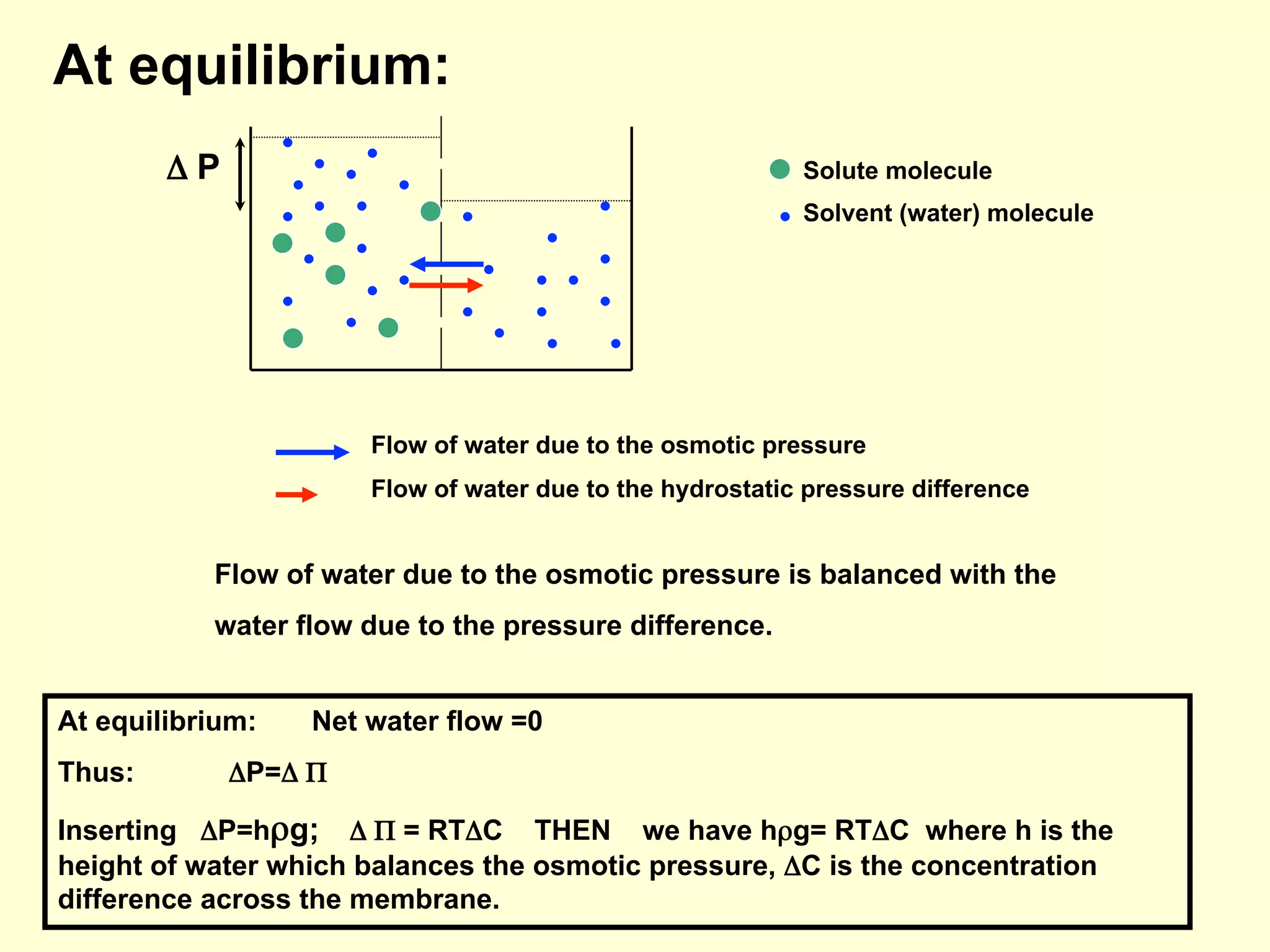
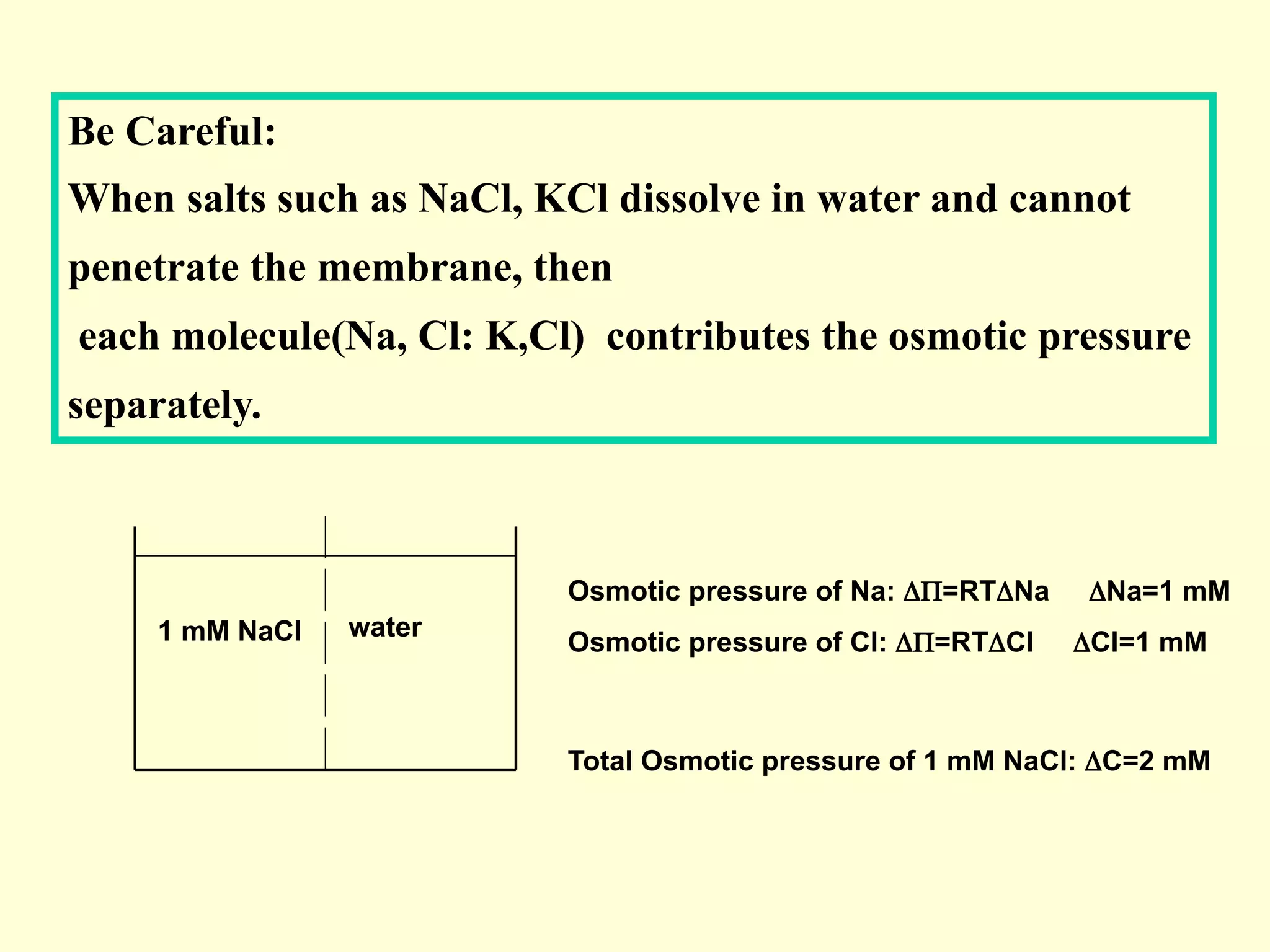
3) Osmosis is the flow of water across a semipermeable membrane from a region of lower solute concentration to higher solute concentration. It creates osmotic pressure that must be balanced by hydrostatic pressure for equilibrium.