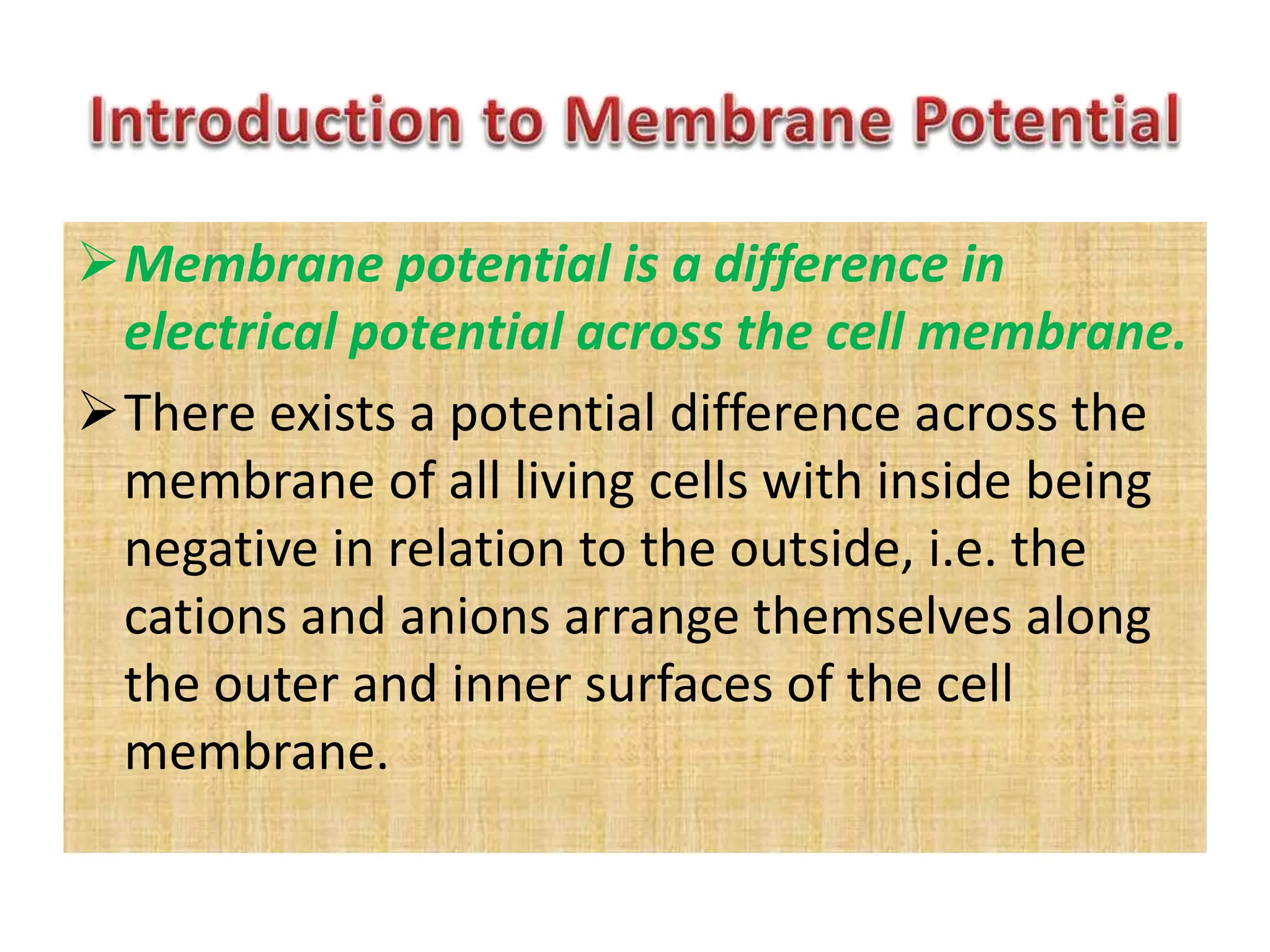
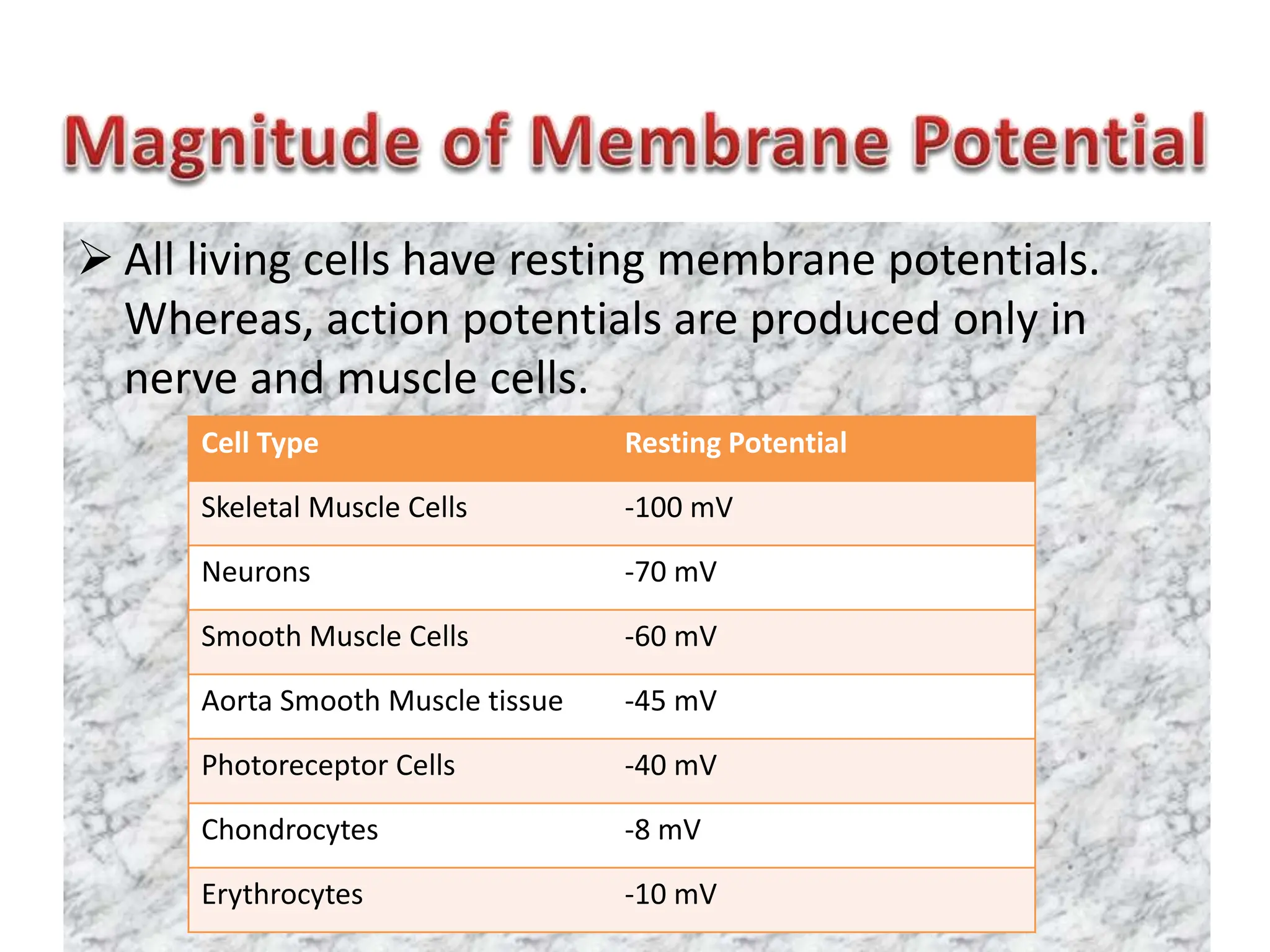
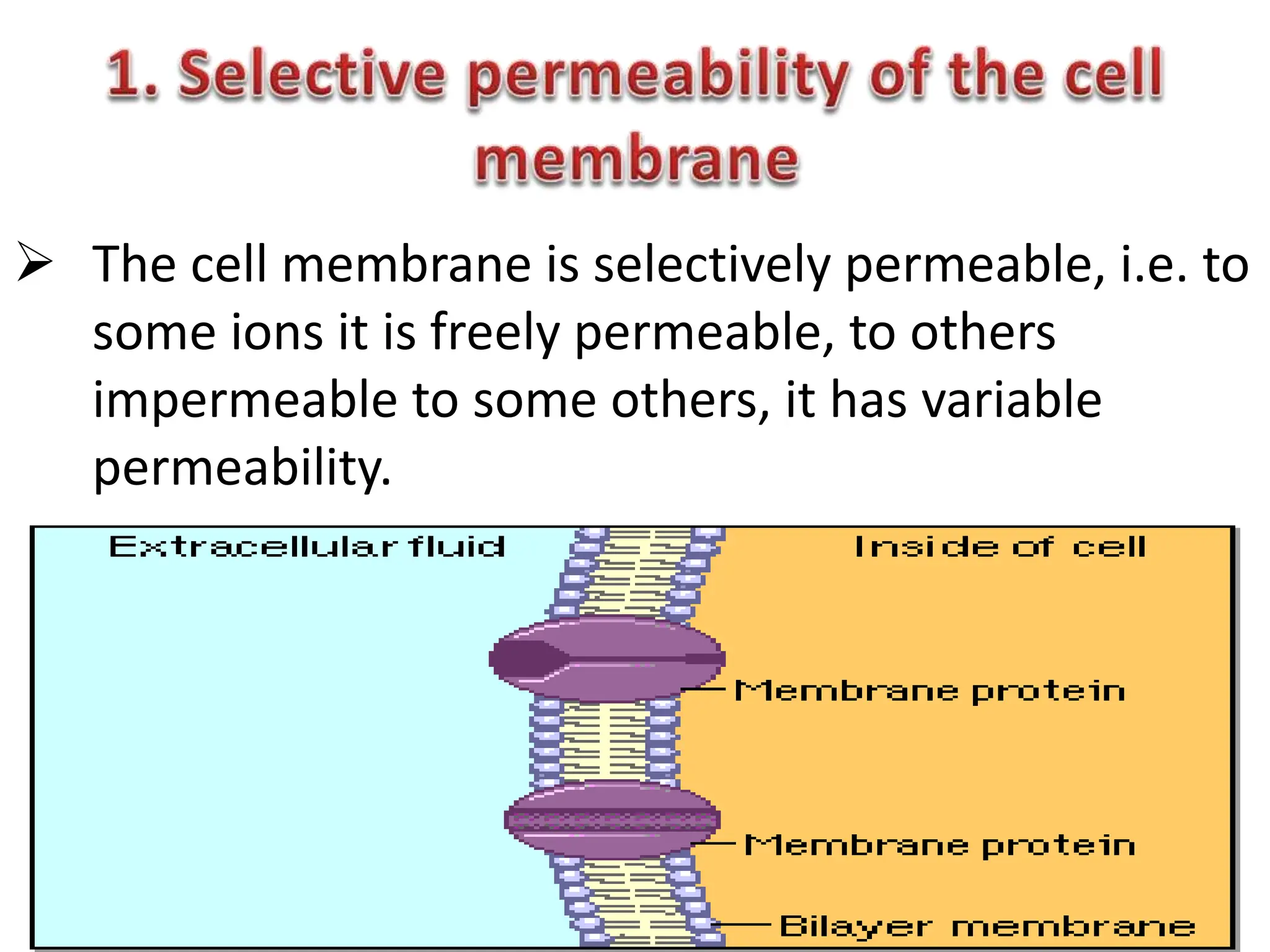
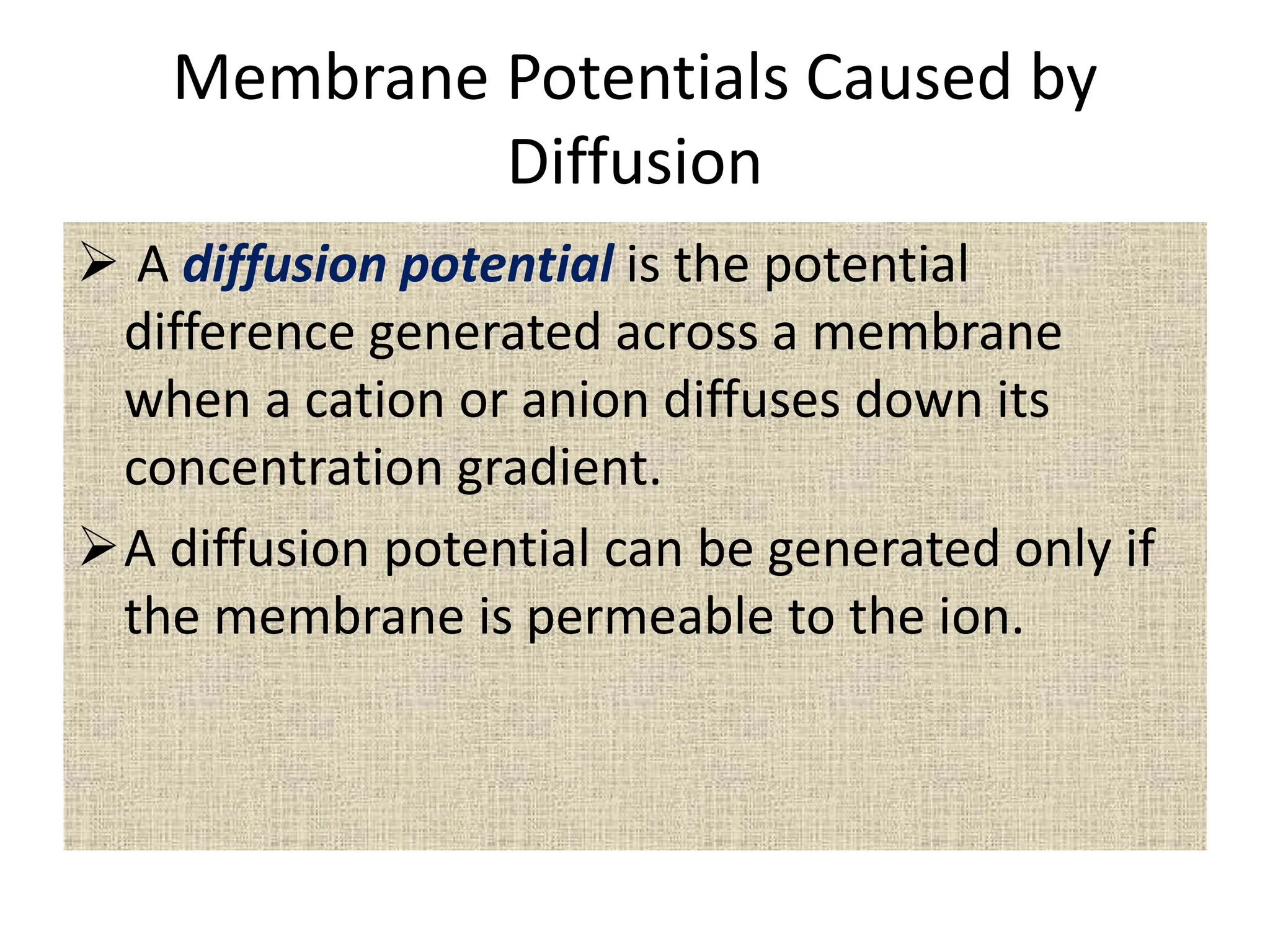
- The membrane potential is caused by a difference in electrical potential across cell membranes, with the inside being negative relative to the outside. This is due to selective permeability of ions like Na+, K+, and Cl- across the membrane.
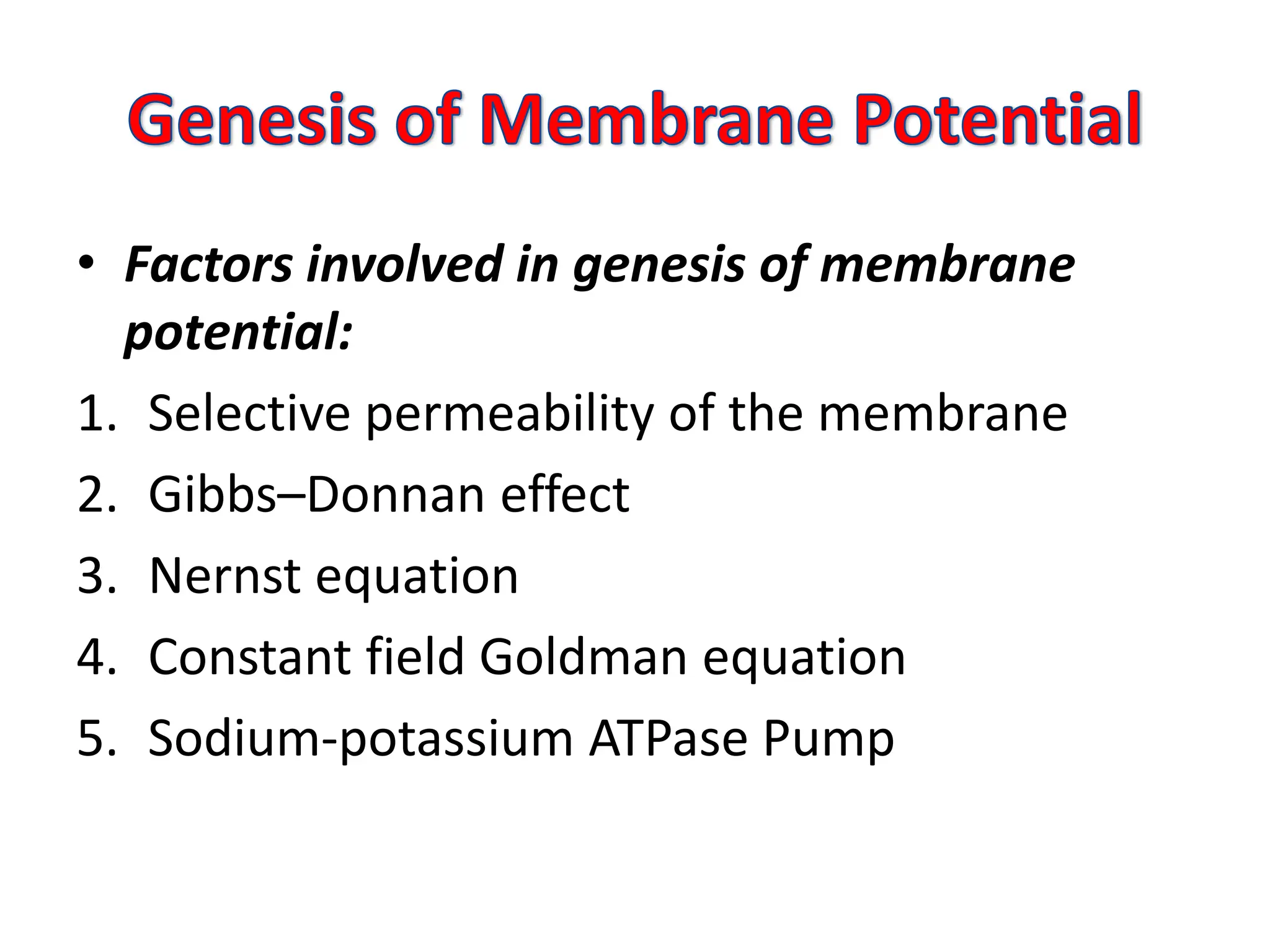
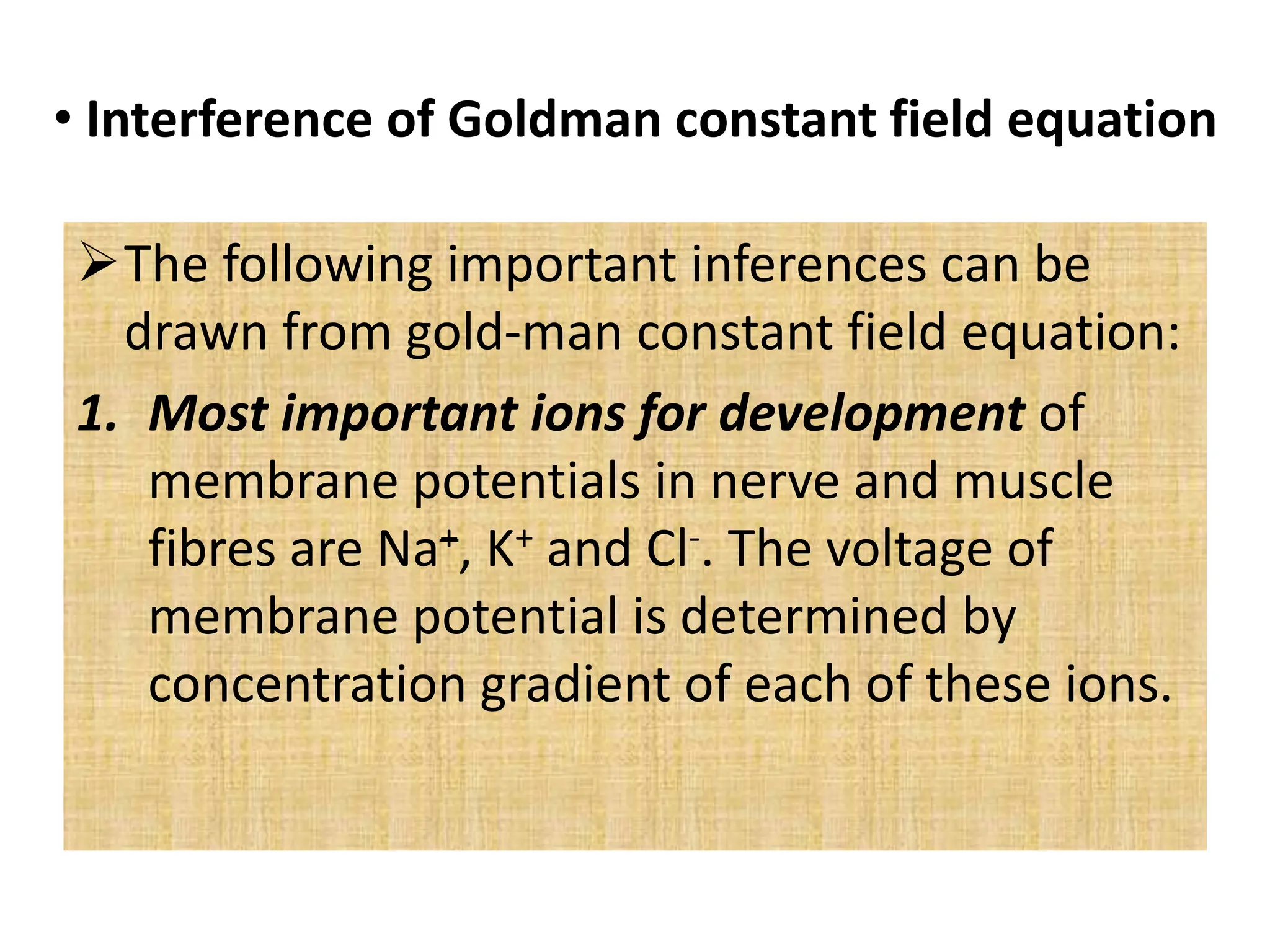
- The Nernst equation and Goldman-Hodgkin-Katz equation can be used to calculate membrane potentials based on ion concentration gradients and permeabilities. Key ions involved are Na+, K+, and Cl-.
- The sodium-potassium pump helps maintain the resting potential by actively transporting more Na+ out and K+ in, making the intracellular side more negative.