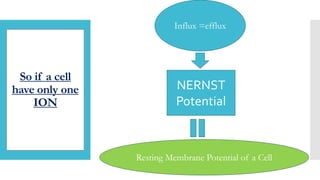
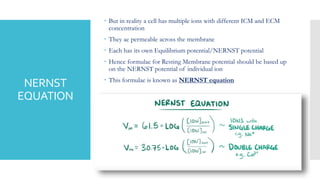
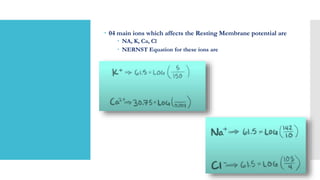
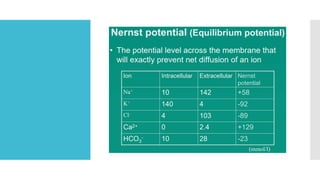
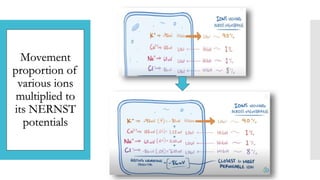
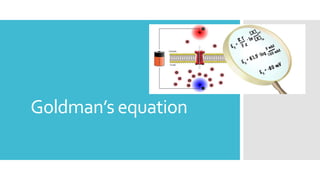
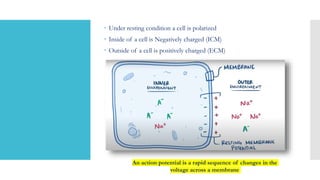
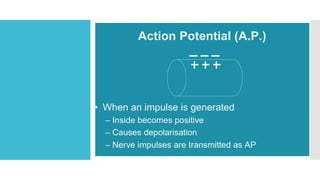
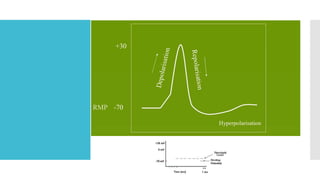
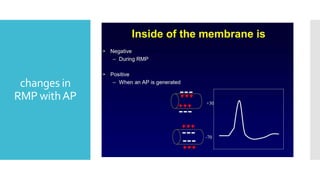
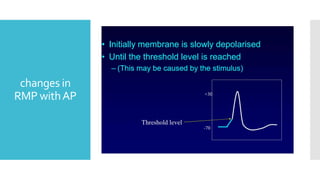
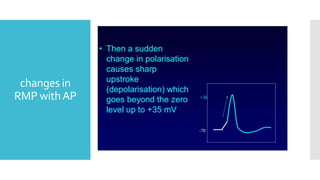
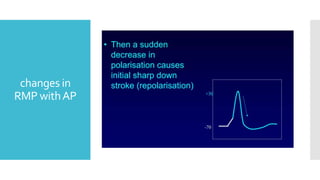
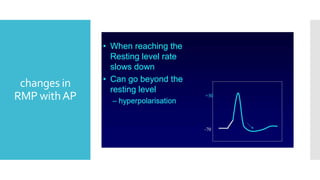
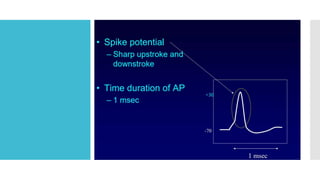
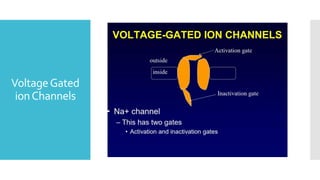
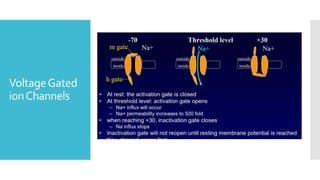
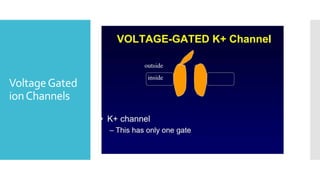
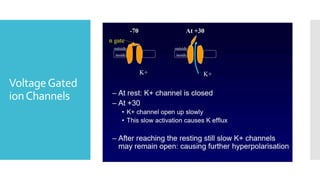
This document provides an overview of membrane potential and action potential, explaining concepts such as resting membrane potential, the role of the sodium-potassium pump, and the Nernst and Goldman equations. It details the factors influencing resting membrane potential, including ion concentration gradients and membrane permeability, as well as the dynamics of action potential. The document emphasizes the variability of resting membrane potential across different cell types and the need for individual ion considerations in calculations.




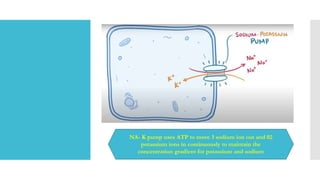
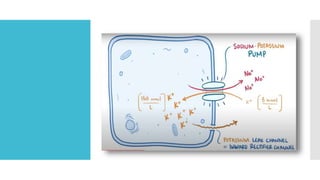
![Sodium
potassium
pump
NA-K pump is a protein that has been
identified in many cells that maintains
the internal concentration
of potassium ions [K+] higher than that in
the surrounding medium (blood, body
fluid, water)
&
Maintains the internal concentration
of sodium ions [Na+] lower than that of
the surrounding medium.
It is an enzyme that uses metabolic energy
to transport (pump) Na+ outward and
K+ inward.
The Resting membrane potential of cells
and related bioelectric phenomena such as
the action potential depend on the steady
state difference in concentrations of
Na+ and K+ maintained by the pump.](https://image.slidesharecdn.com/2-230926070140-cf0f4899/85/2-BIOPHYSICS-Membrane-Potentials-and-Action-Potential-pptx-11-320.jpg)