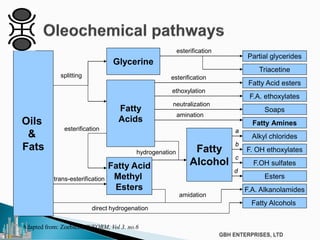
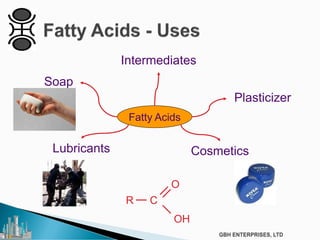
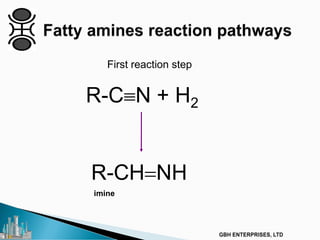
The document outlines various chemical intermediates and products derived from natural triglycerides, including the five basic oleochemicals: fatty acids, fatty alcohols, fatty methyl esters, fatty amines, and glycerine. It discusses the sources of these materials, the processes for their production such as esterification and hydrogenation, and the role of catalysts in these processes. Additionally, it highlights the growing demand for biodiesel and other applications in personal care and pharmaceuticals, emphasizing the importance of operational and financial management in production.