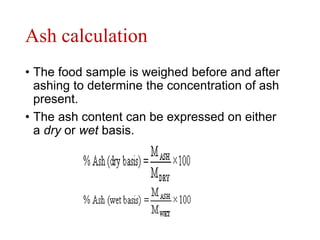
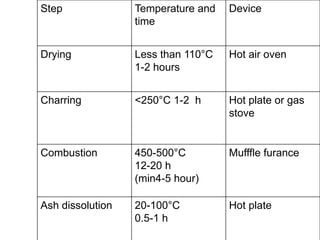
This document discusses methods and techniques for ashing samples to determine their mineral content. It describes:
1) Dry ashing, which uses high temperatures in a muffle furnace to burn off organic matter, leaving mineral residues. Most minerals are converted to oxides, sulfates, or silicates.
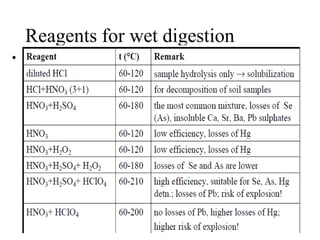
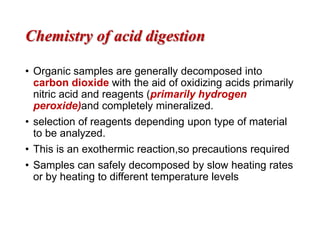
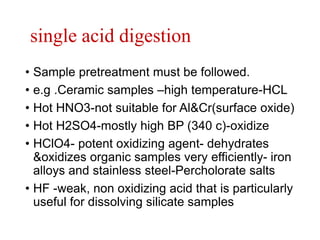
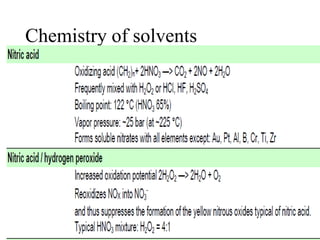
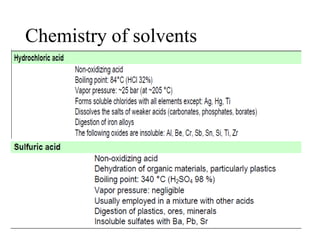
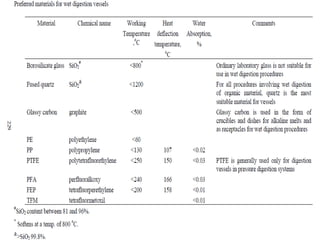
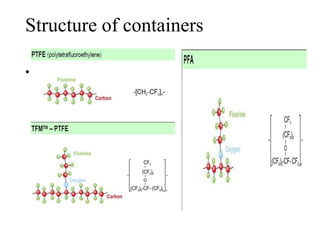
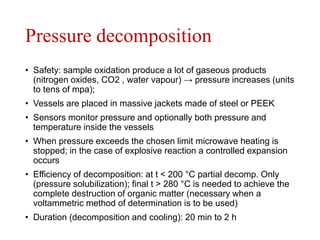
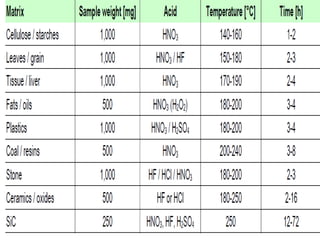
2) Wet ashing, which uses acids and oxidizing agents to break down organic matrices and leave minerals in aqueous solution for analysis of specific minerals.
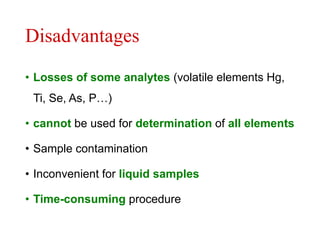
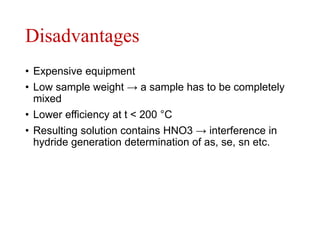
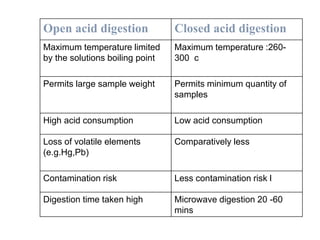
3) Key steps for both methods including sample preparation, ashing procedures, and advantages like efficiency while avoiding volatile element losses. Contamination risks and time requirements are disadvantages.