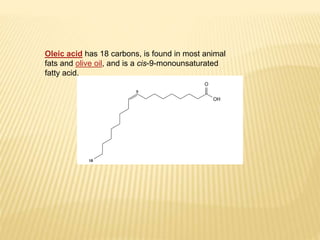
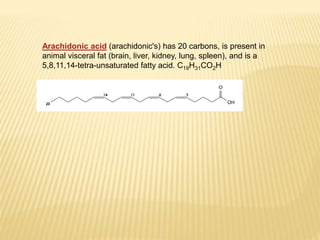
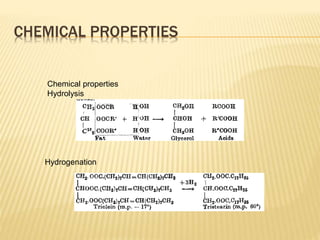
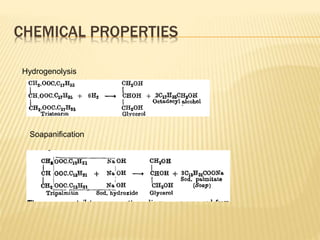
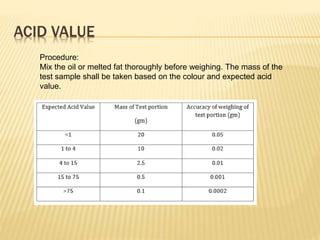
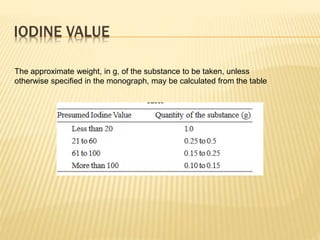
Fats and oils are composed of triglycerides, which are esters of fatty acids and glycerol. They can be saturated or unsaturated depending on the number of double bonds in the fatty acid chains. Trans fats are unsaturated fats that have been hydrogenated, changing the geometry of double bonds from cis to trans. Partial hydrogenation of oils reduces unsaturation and makes them less nutritious. Tests like acid value, saponification value, iodine value and acetyl value are used to analyze and characterize fats and oils based on their properties.




![Unsaturated fatty acids generally have cis configurations as
opposed to trans configurations.While saturated fatty acids
without any double bonds are created from unsaturated fats by
the process of fat hydrogenation, partial hydrogenation
converts some of the cis double bonds into trans double bonds
by an isomerization reaction with the catalyst used for the
hydrogenation, which yields a trans fat.[3][4]
Double bonds may be in either a cis or a trans isomer, depending
on the geometry of the double bond. In the cis isomer, hydrogen
atoms are on the same side of the double bond; whereas in the
trans isomer, they are on opposite sides of the double bond
FATS AND OILS](https://image.slidesharecdn.com/fatsandoils-230409040445-de293626/85/FATS-AND-OILS-pptx-5-320.jpg)