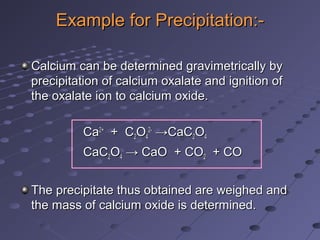
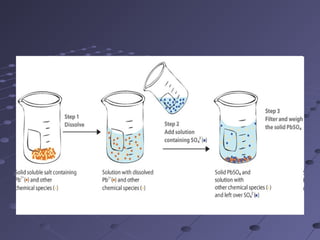
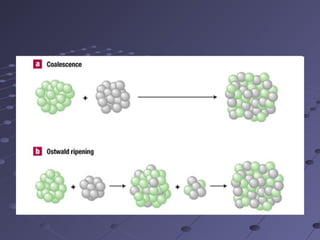
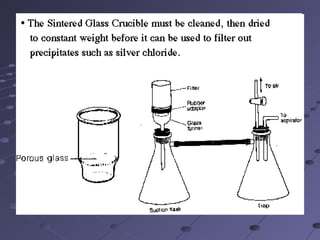
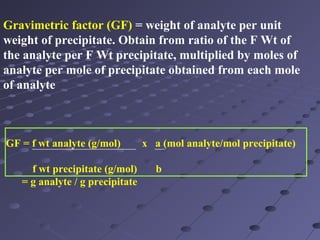
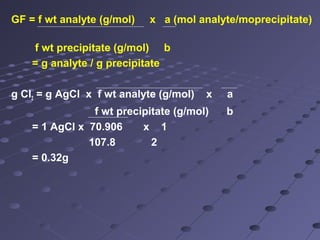
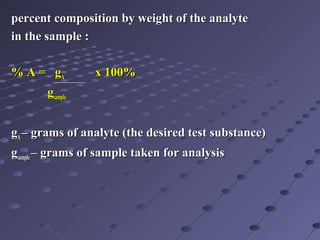
Gravimetric analysis involves determining the amount of analyte by measuring the mass of a pure substance containing the analyte. It has two main types - precipitation and volatilization. In precipitation, the analyte is converted to a solid precipitate using a reagent, then the precipitate is filtered, washed, purified and weighed. In volatilization, the analyte or its products are vaporized and collected or the mass loss is measured. Key steps in gravimetric analysis include preparation of the analyte solution, precipitation, digestion, filtration, washing, drying/ignition, and weighing to calculate the analyte amount. It is potentially more accurate and precise than volumetric analysis but requires proper technique.


















![Orthophosphate (POOrthophosphate (PO44
3-3-
) is determined by) is determined by
weighing as ammonium phosphomolybdateweighing as ammonium phosphomolybdate
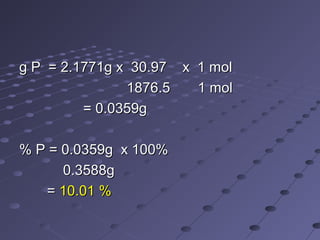
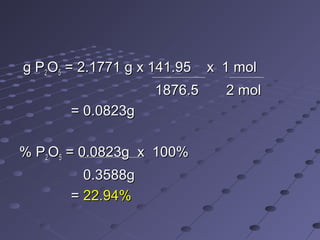
(NH(NH44)PO)PO44.12MoO.12MoO33. Calculate the percent P. Calculate the percent P
and the percent Pand the percent P22OO55 if 2.1771g precipitateif 2.1771g precipitate
(ppt) were obtained from a 0.3588g sample.(ppt) were obtained from a 0.3588g sample.
[F wt P = 30.97], [F wt P.molybdate = 1876.5],[F wt P = 30.97], [F wt P.molybdate = 1876.5],
[F wt P[F wt P22OO55 = 141.95]= 141.95]](https://image.slidesharecdn.com/chemmaths1-150629222436-lva1-app6892/85/Chem-maths1-27-320.jpg)