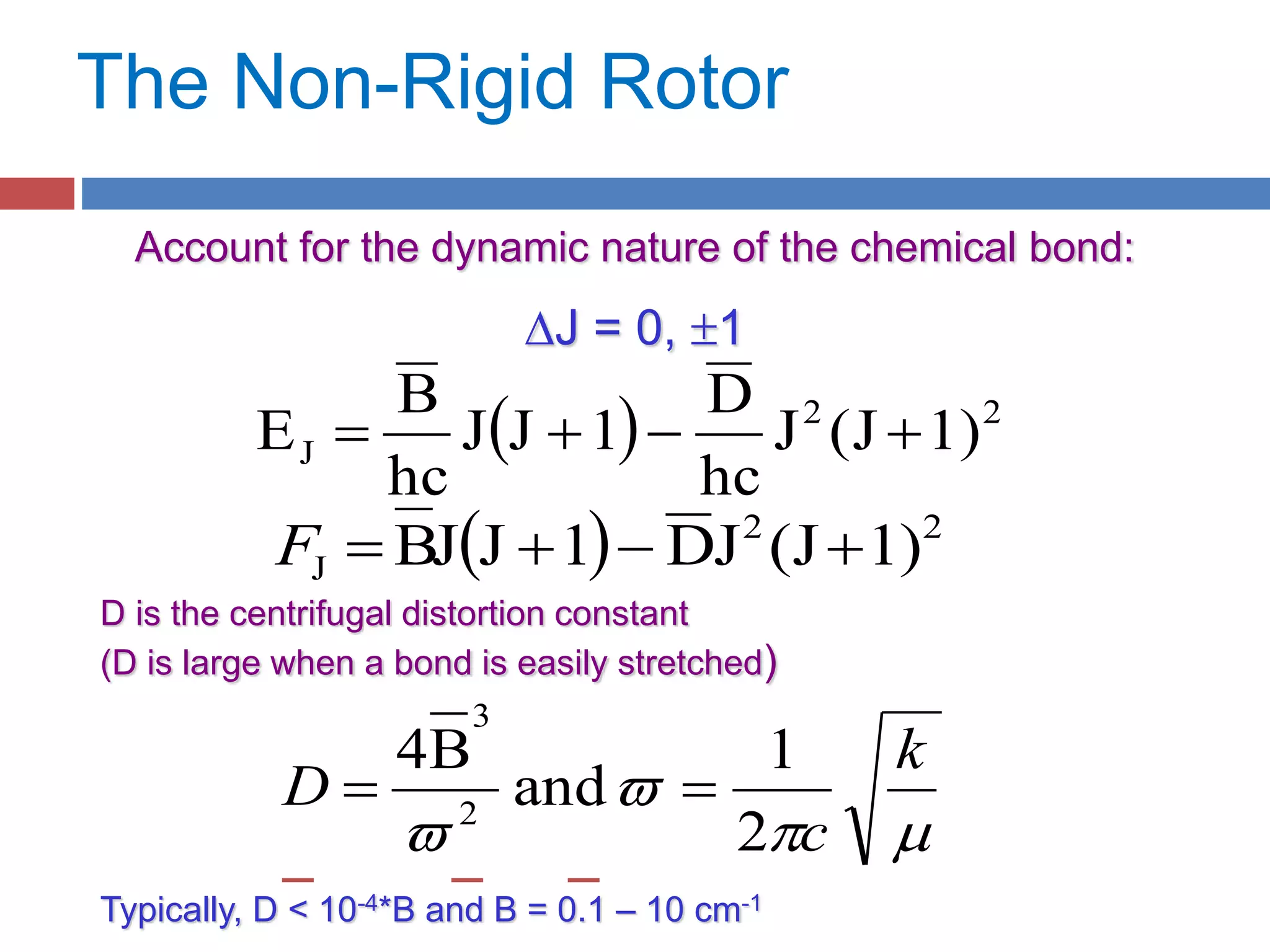
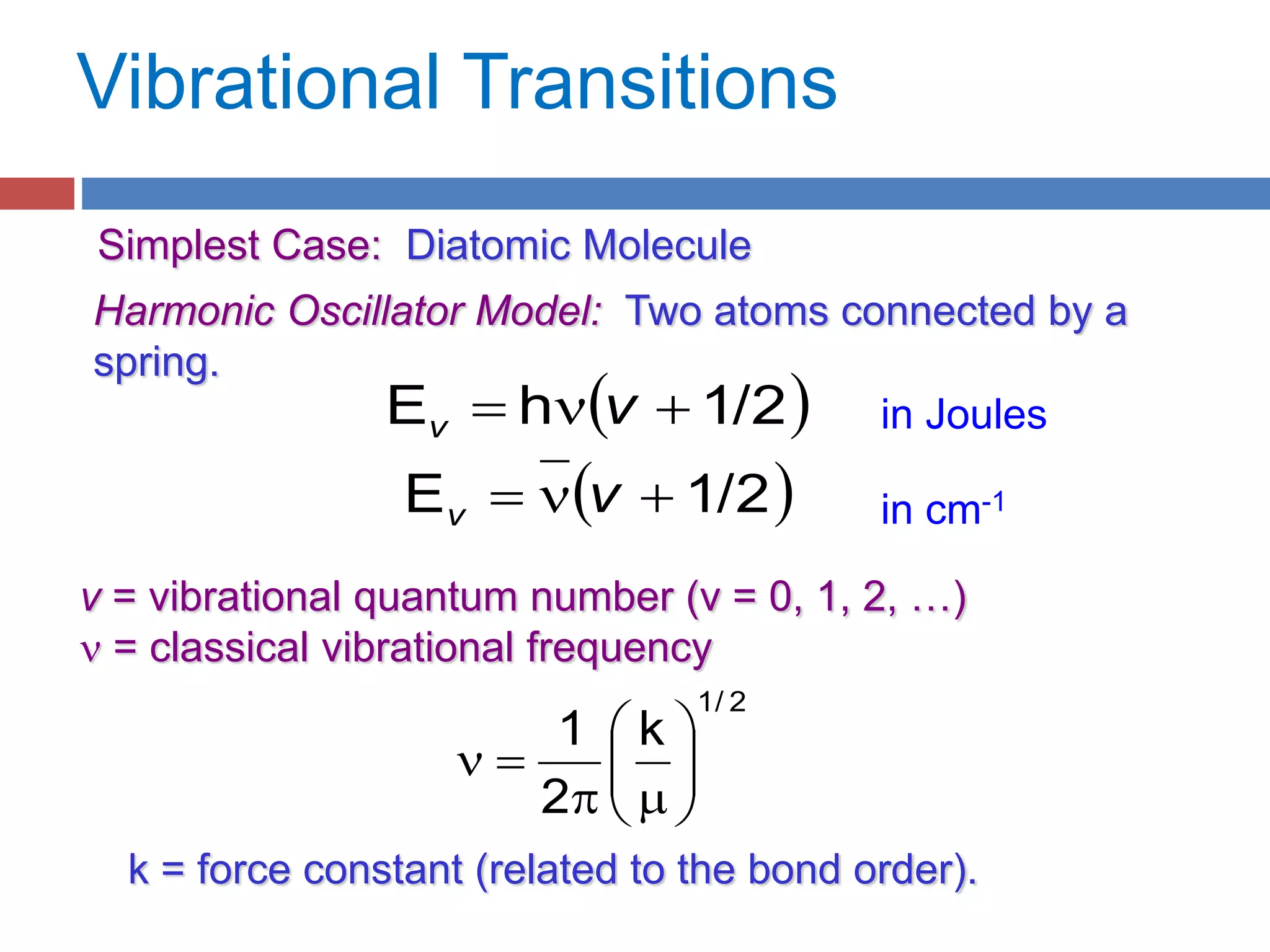
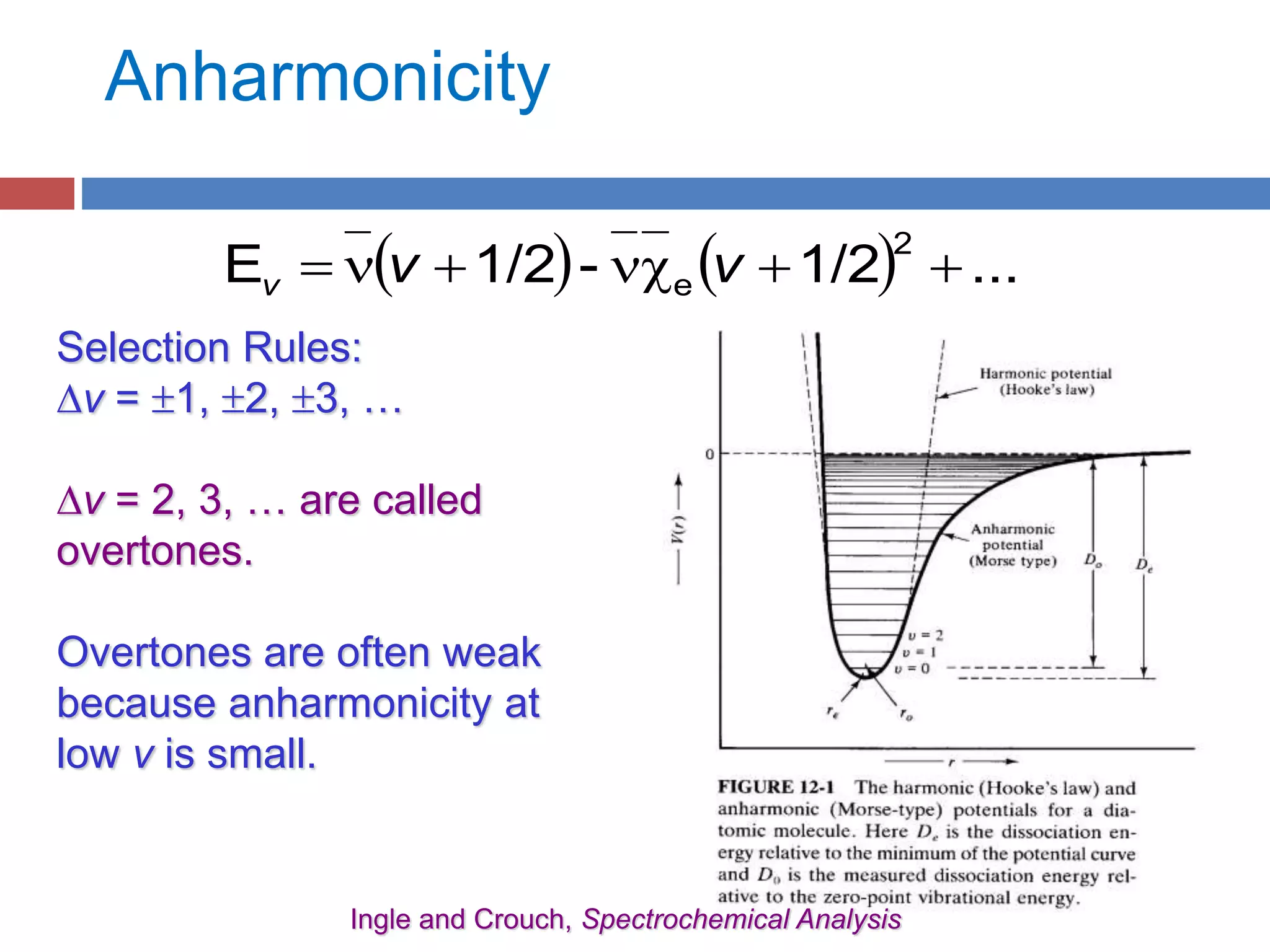
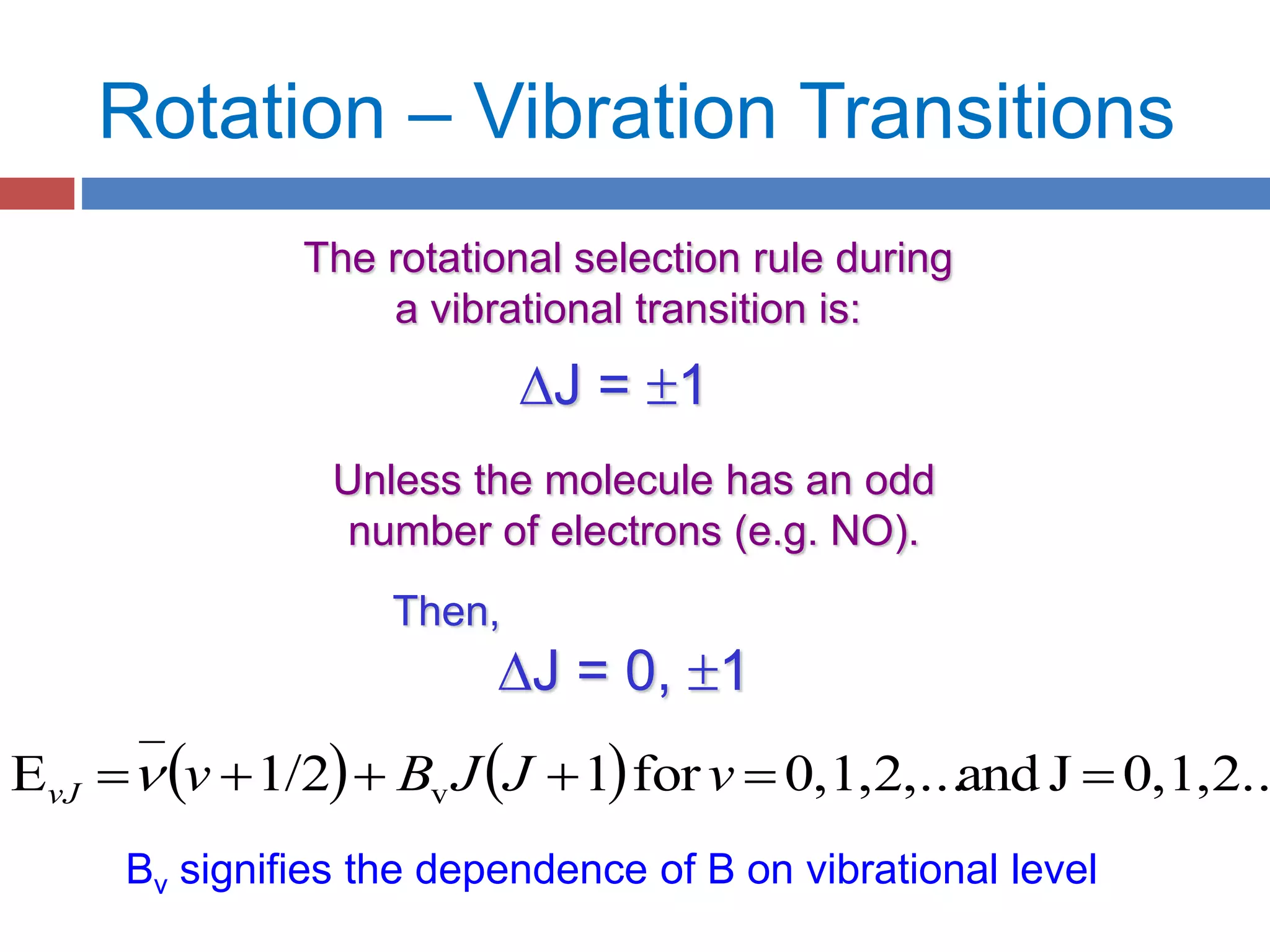
Rotational spectroscopy measures the energies of rotational states of molecules. It can observe the rotation of polar molecules using microwave or infrared spectroscopy, and of non-polar molecules using Raman spectroscopy. Molecules can be modeled as rigid or non-rigid rotors. Diatomic and linear molecules can be modeled as rigid rotors, while distortions are accounted for in non-rigid rotor models. Vibrational states are modeled as harmonic oscillators, though anharmonicity is considered. Rotational and vibrational states are quantized, and selection rules apply to rotational-vibrational transitions.