Embed presentation
Downloaded 62 times


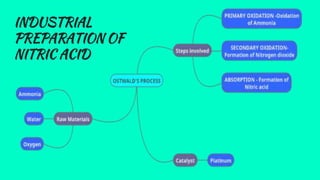
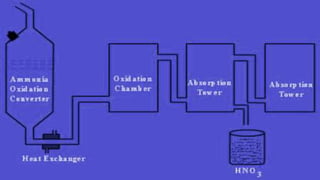
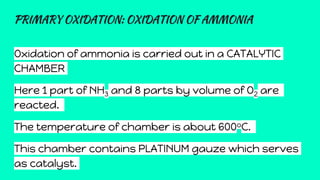
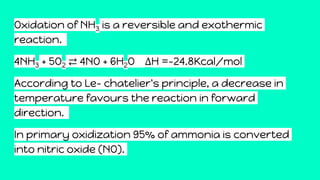






Nitric acid is produced through a multi-step industrial process involving the oxidation of ammonia. Ammonia is oxidized to nitric oxide at high temperatures in the presence of a platinum catalyst. The nitric oxide is then cooled and further oxidized to nitrogen dioxide, which is absorbed in water to form nitric acid. The dilute nitric acid is concentrated through reabsorption, resulting in a 68% concentrated solution used in fertilizer production, explosives, and other applications requiring a strong oxidizing acid.










