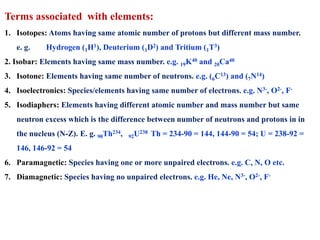
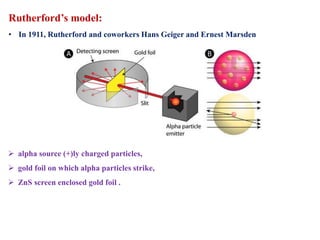
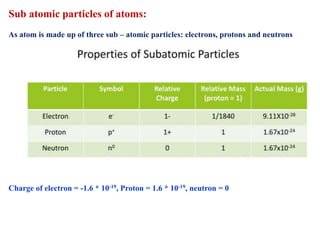
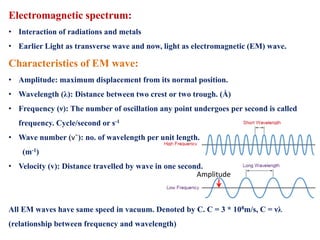
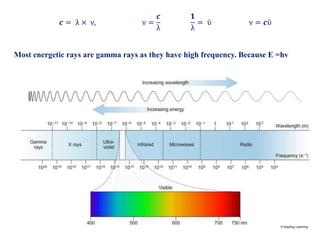
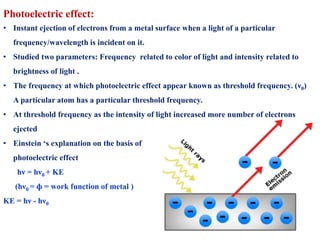
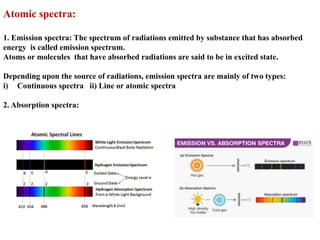
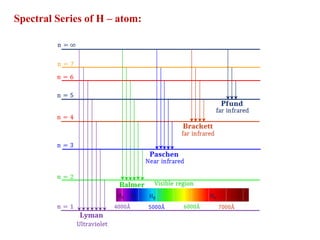
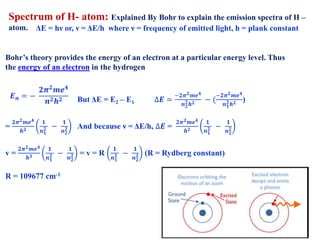
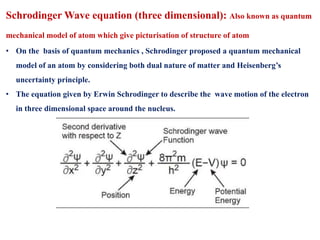
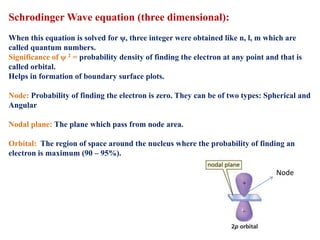
This document defines various terms associated with elements and their subatomic particles. It then summarizes Rutherford's gold foil experiment and conclusions that led to the nuclear model of the atom. The document continues by describing the key subatomic particles (protons, neutrons, electrons), electromagnetic spectrum, photoelectric effect, atomic spectra, Bohr's model of the hydrogen atom, de Broglie wavelength, Heisenberg's uncertainty principle, Schrodinger wave equation, shapes of orbitals, filling of orbitals according to Aufbau principle and Hund's rule.



![9. The orbital diagram in which the Aufbau Principle is violated:
10. The outermost electronic configuration of most electronegative element is:
a) ns2np3 b) ns2np4 c) ns2np5 ns2np6
11. The correct G. S. electronic configuration of Cr atom is:
a) [Ar]3d54s1 b) [Ar]3d44s2 c) [Ar] 3d64s0 d) [Ar]4d55s1](https://image.slidesharecdn.com/atomicstructurepresentation-200622062247/85/Atomic-structure-presentation-21-320.jpg)



