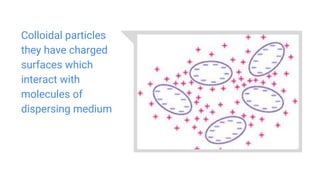
The document explains osmotic pressure, which is the pressure needed to halt osmosis, and details the classifications of tonicity: isotonic, hypertonic, and hypotonic. It discusses the applications of osmotic pressure in biochemistry, plant water transport, and food preservation, as well as reverse osmosis for filtration and desalination. Additionally, the document covers colloids, their types, properties, and their behavior in light, alongside the definition and properties of suspensions.