Transition metals such as manganese, iron, and copper can exist in multiple oxidation states. Manganese commonly exists as Mn2+, Mn4+, and Mn7+. Potassium manganate (VII), KMnO4, is a powerful oxidizing agent. Iron exists as Fe2+ and Fe3+ and acts as a catalyst in the Haber process. Copper exists as Cu+ and Cu2+. Compounds of Cu+ are generally colorless and insoluble while compounds of Cu2+ are blue and soluble, forming complexes with ligands like OH-, NH3, and CO32-.



![ Reduction of KMnO4 in Alkaline Medium:
2KMnO4 + 2KOH 2K2MnO4 + H2O + O
Purple Green
Mn = +7 Mn = +6
2K2MnO4 + 2KOH 2MnO2 + 4KOH + 2O
Green Brown
Mn = +6 Mn= +4
Reduction of KMnO4 in acidic medium:
2KMnO4 + 3H2SO4 (dilute) K2SO4 + 2MnSO4 + 3H2O + 5[O]
2MnO4
- + 6H+ 2Mn+2 + 3H2O + 5[O]
Purple Colorless
Mn = +7 Mn = +2](https://image.slidesharecdn.com/transitionmetalsmn-171204172901/75/Transition-metals-Manganese-Iron-and-Copper-4-2048.jpg)
![Reactions of Manganese (II) ions in solution
1. The reaction of Hexaaquomanganese (II) ions with hydroxide ions:
OH- ions remove H+ from two of the water ligands to give a neutral complex.
This is insoluble in water and a precipitate is formed.
[Mn(H2O)6]+2 + 2OH- [Mn(H2O)4(OH)2] + 2H2O](https://image.slidesharecdn.com/transitionmetalsmn-171204172901/75/Transition-metals-Manganese-Iron-and-Copper-5-2048.jpg)
![2. The reaction of Hexaaquomanganese (II) ions with ammonia solution:
Ammonia removes H+ ions from the aqua complex
[Mn(H2O)6]+2 + 2NH3 [Mn(H2O)4(OH)2] + 2NH4
+](https://image.slidesharecdn.com/transitionmetalsmn-171204172901/75/Transition-metals-Manganese-Iron-and-Copper-6-2048.jpg)

![In testing for a Double Bond
1. In testing for H2C=CH2
2KMnO4 + H2O 2MnO2 + 2KOH + 3[O]
3x{ H2C=CH2 + O + H2O HO-CH2-CH2-OH }
2KMnO4 + 4H2O + 3H2C=CH2 2MnO2 + 2KOH + 3HO-CH2-CH2-OH
2. Reaction of Hydrazine with KMnO4 (N=-2 to N = 0)
2x{ 2KMnO4 + 3H2SO4 K2SO4 + 2MnSO4 + 3H2O + 5[O] }
5x{ N2H4 + 2[O] N2 + 2H2O }
4KMnO4 + 6H2SO4 + 5N2H4 2K2SO4 + 4MnSO4 + 16H2O + 5N2](https://image.slidesharecdn.com/transitionmetalsmn-171204172901/75/Transition-metals-Manganese-Iron-and-Copper-8-2048.jpg)

![Using KMnO4 as an oxidizing agent in
Titrations
Potassium manganate(VII) solution is use to find the concentration
of all sorts of reducing agents. It is always used in acidic solution.
Examples:
1. Oxidation of Fe+2 to Fe+3
2KMnO4 + 3H2SO4 K2SO4 + 2MnSO4 + 3H2O + 5[O]
5x{ 2FeSO4 + H2SO4 + [O] Fe2(SO4)3 + H2O}
2KMnO4 + 8H2SO4 + 10 FeSO4 K2SO4 + MnSO4 + 5Fe2(SO4)3 + 8H2O](https://image.slidesharecdn.com/transitionmetalsmn-171204172901/75/Transition-metals-Manganese-Iron-and-Copper-10-2048.jpg)
![2. Ethanedioic acid (Oxalic Acid) (C=+3) to Carbon Dioxide (C=+4)
2KMnO4 + 3H2SO4 K2SO4 + 2MnSO4 + 3H2O + 5[O]
5x{ H2C2O4 + [O] 2CO2 + 2H2O }
2KMnO4 + 3H2SO4 + 5H2C2O4 K2SO4 + 2MnSO4 + 8H2O + 10CO2
3. Sulphite ions (S=+4) to Sulphate ions (S=+6)
2KMnO4 + 3H2SO4 K2SO4 + 2MnSO4 + 3H2O + 5[O]
5x{ Na2SO3 + [O] Na2SO4 }
2KMnO4 + 3H2SO4 + 5Na2SO3 K2SO4 + 2MnSO4 + 3H2O + 5Na2SO4
4. Potassium ferrocyanide (Fe= +2) to Potassium ferricyanide (Fe= +3)
2KMnO4 + 3H2SO4 K2SO4 + 2MnSO4 + 3H2O + 5[O]
5x{ 2K4[Fe(CN)6] + O + H2O 2K3[Fe(CN)6] + 2KOH }
2KMnO4 + 3H2SO4 + 10K4[Fe(CN)6] K2SO4 + 2MnSO4 + 2H2O + 10K3[Fe(CN)6] + 10KOH](https://image.slidesharecdn.com/transitionmetalsmn-171204172901/75/Transition-metals-Manganese-Iron-and-Copper-11-2048.jpg)







![b. Reaction of Iron ions with NH3
In case of Iron (II) In case of Iron (III)
Ammonia acts a base and removes H+ ions from the water ligands to
form a neutral insoluble complex.
[Fe(H2O)6]+3 + 3NH3 [Fe(H2O)3(OH)3] + 3NH4
+[Fe(H2O)6]+2 + 2NH3 [Fe(H2O)4(OH)2] + 2NH4
+](https://image.slidesharecdn.com/transitionmetalsmn-171204172901/75/Transition-metals-Manganese-Iron-and-Copper-19-2048.jpg)

![b. Iron (III) ions with Carbonate ions
The hexaaquairon(III) ion is sufficiently acidic to react with weakly
basic carbonate ions.
Carbonate ions remove H+ from water ligands to produce a neutral
complex
2[Fe(H2O)6]+3 + 3CO3
-2 2[Fe(H2O)3(OH)3] + 3CO2 + 3H2O](https://image.slidesharecdn.com/transitionmetalsmn-171204172901/75/Transition-metals-Manganese-Iron-and-Copper-21-2048.jpg)
![Testing for Iron(III) with Thiocyanate ions
Add thiocyanate ions, SCN-, to a solution containing
iron(III) ions, blood red solution is formed.
[Fe(H2O)6]+3 + SCN- [Fe(SCN)(H2O)5]+2 + H2O](https://image.slidesharecdn.com/transitionmetalsmn-171204172901/75/Transition-metals-Manganese-Iron-and-Copper-22-2048.jpg)



![Compounds of Cu+2
Most of the anhydrous cupric compounds are colorless while the hydrated
cupric compounds are blue due to the formation of Blue hydrated ion
[Cu(H2O)4]+2 or [Cu(H2O)6]+2
Solutions of Cu+2 salts are acidic in nature due to hydrolysis.
Cu+2 + H2O [Cu(OH)]+ + H+
Since the outer most shell of Cu+2 ion has one unpaired electron (3d9), Cu+2
compounds are paramagnetic.](https://image.slidesharecdn.com/transitionmetalsmn-171204172901/75/Transition-metals-Manganese-Iron-and-Copper-26-2048.jpg)
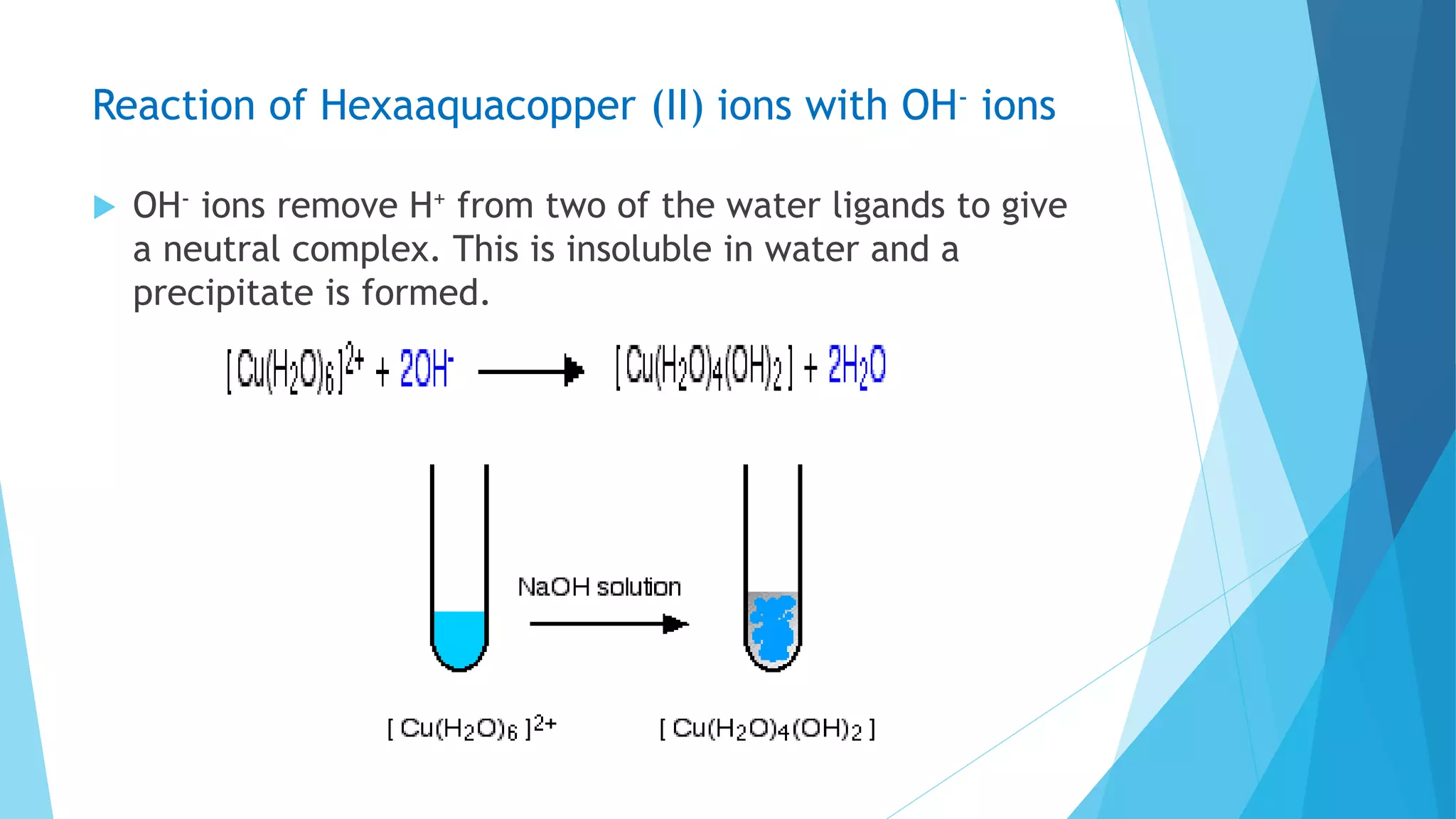
![Reaction of Hexaaquacopper (II) ion with NH3 solution
When Cu(OH)2 is dissolved in excess of NH4OH, a blue
soluble cupric ammine hydroxide is obtained
Cu(OH)2 + 4NH4OH [Cu(NH3)4(OH)2] + 4H2O](https://image.slidesharecdn.com/transitionmetalsmn-171204172901/75/Transition-metals-Manganese-Iron-and-Copper-28-2048.jpg)

