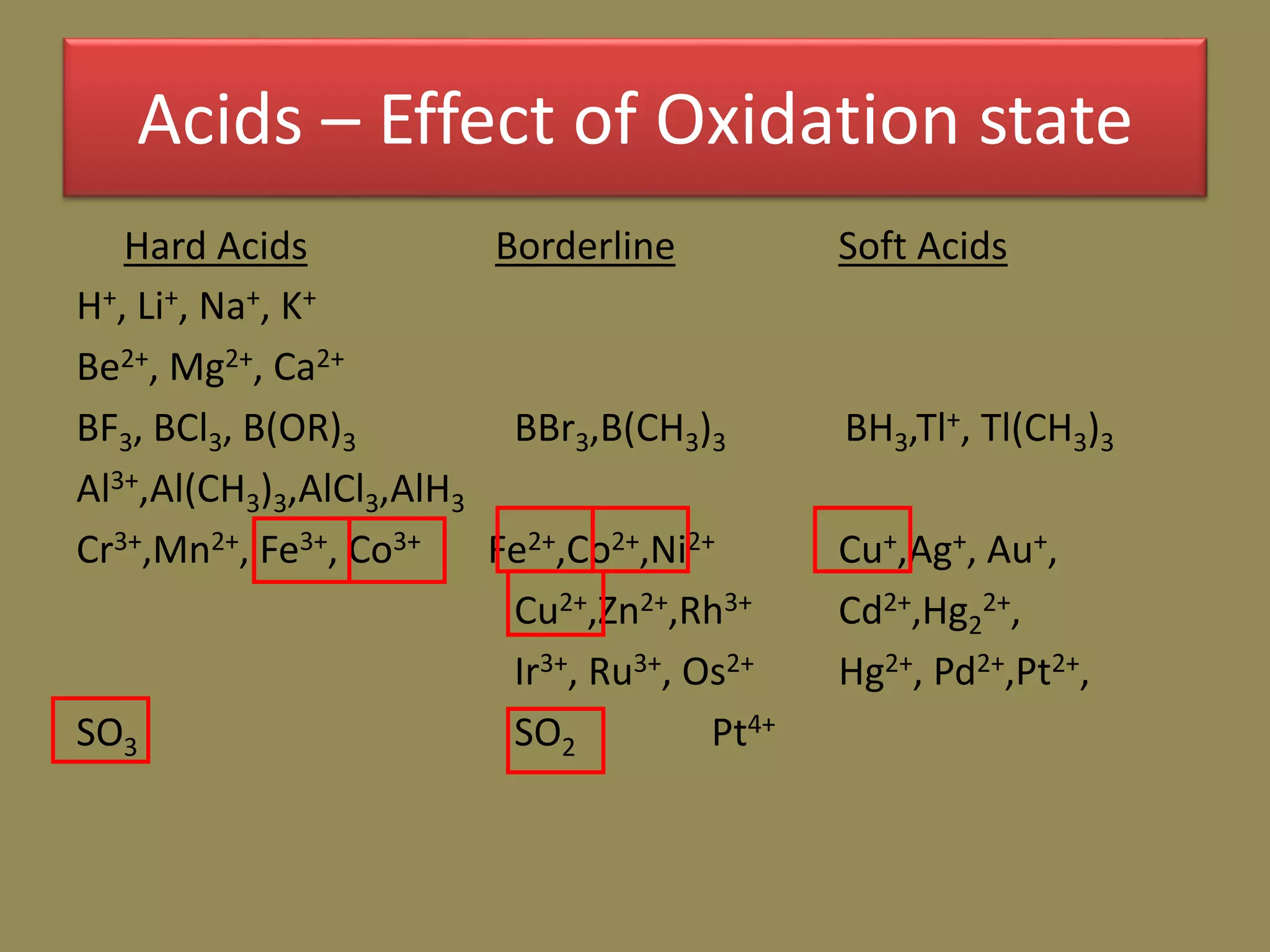
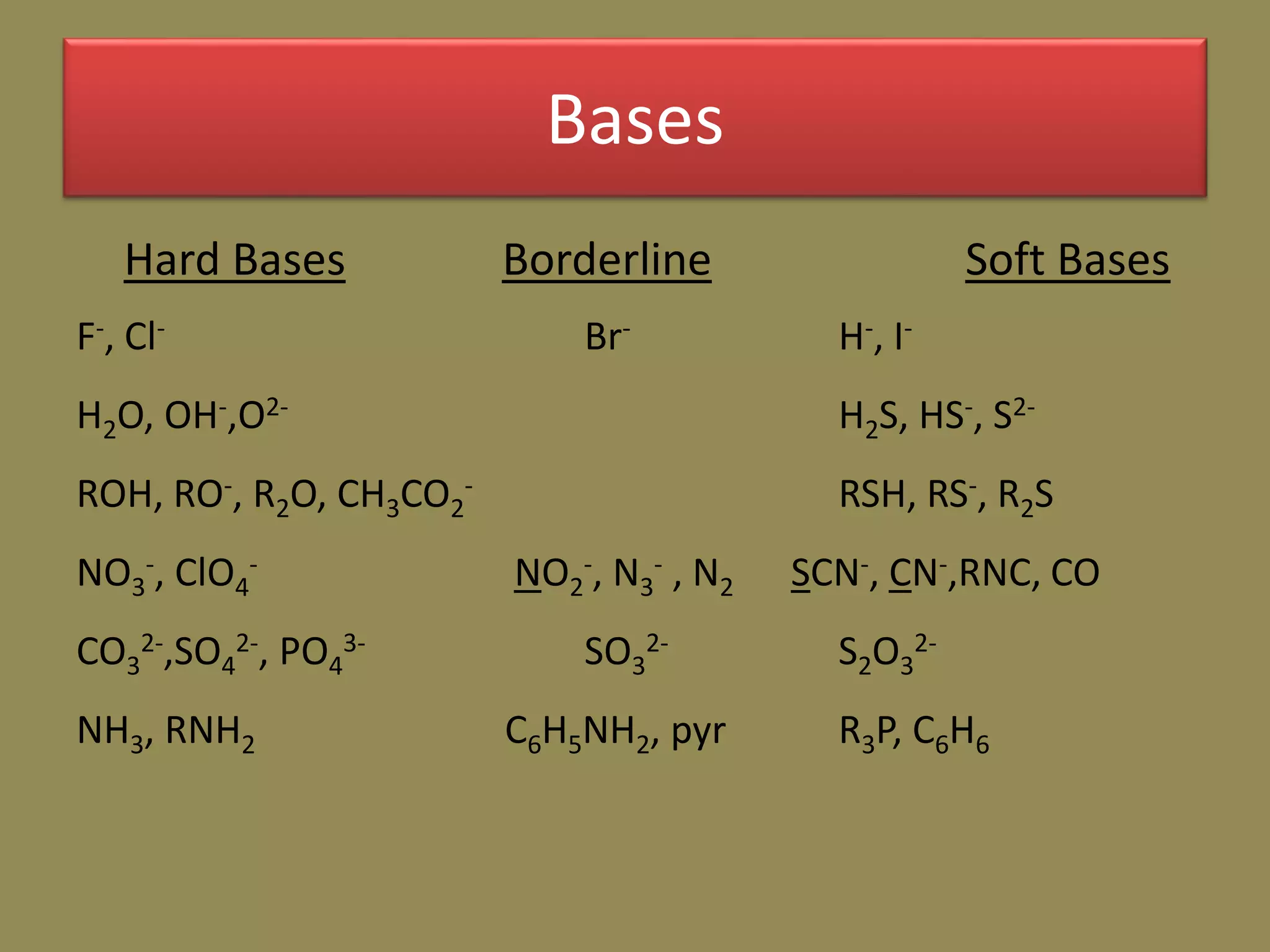
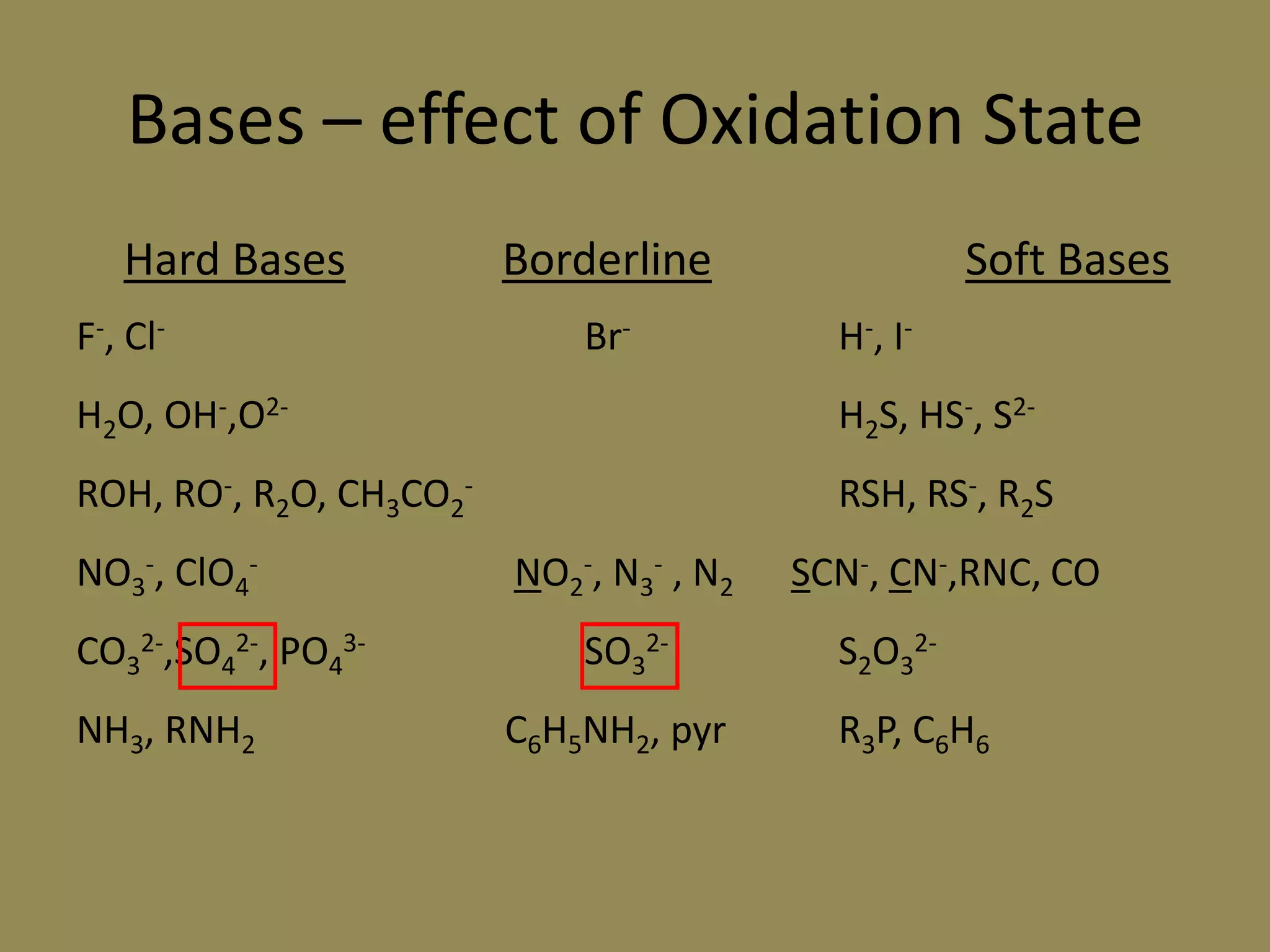
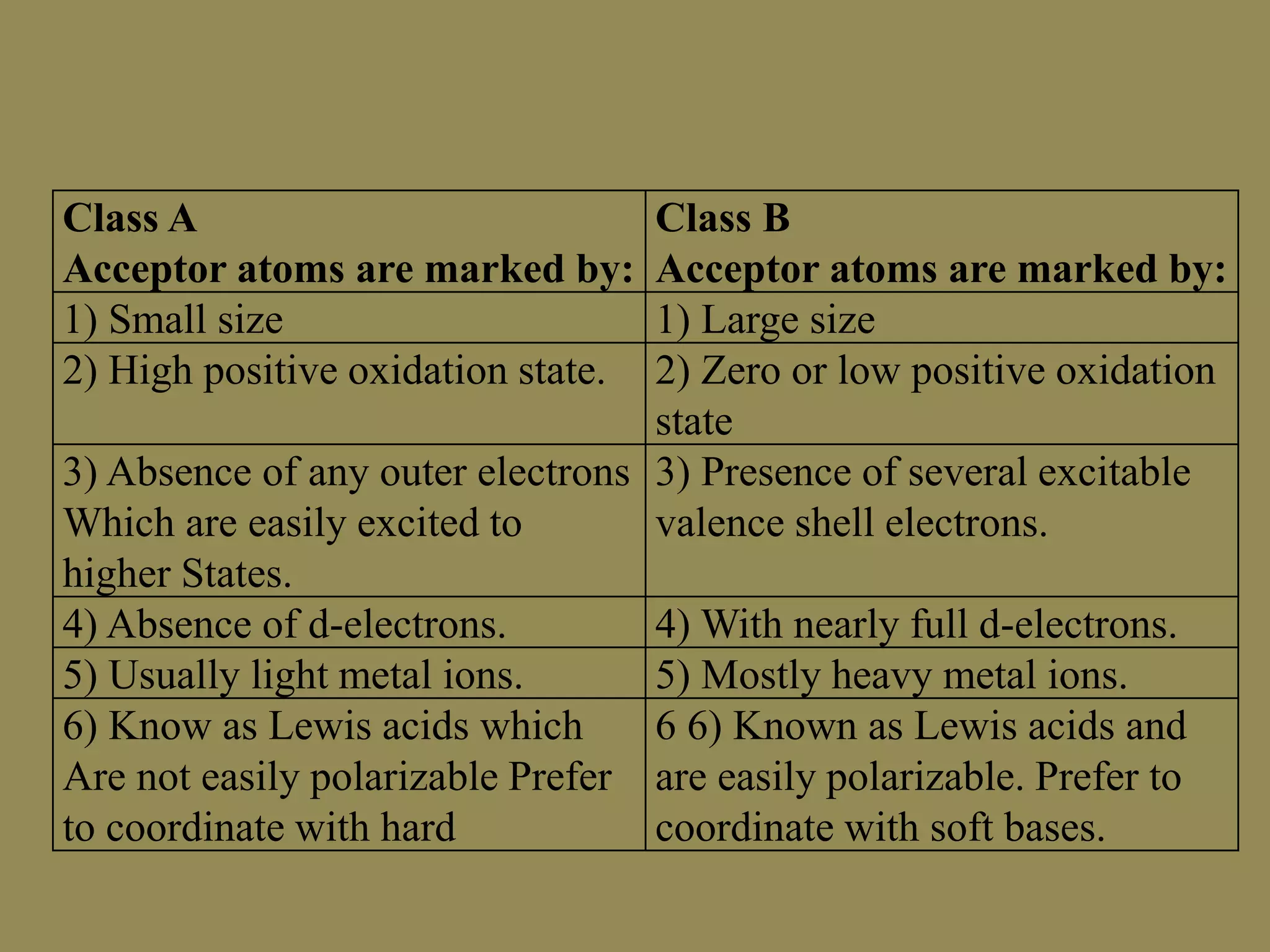
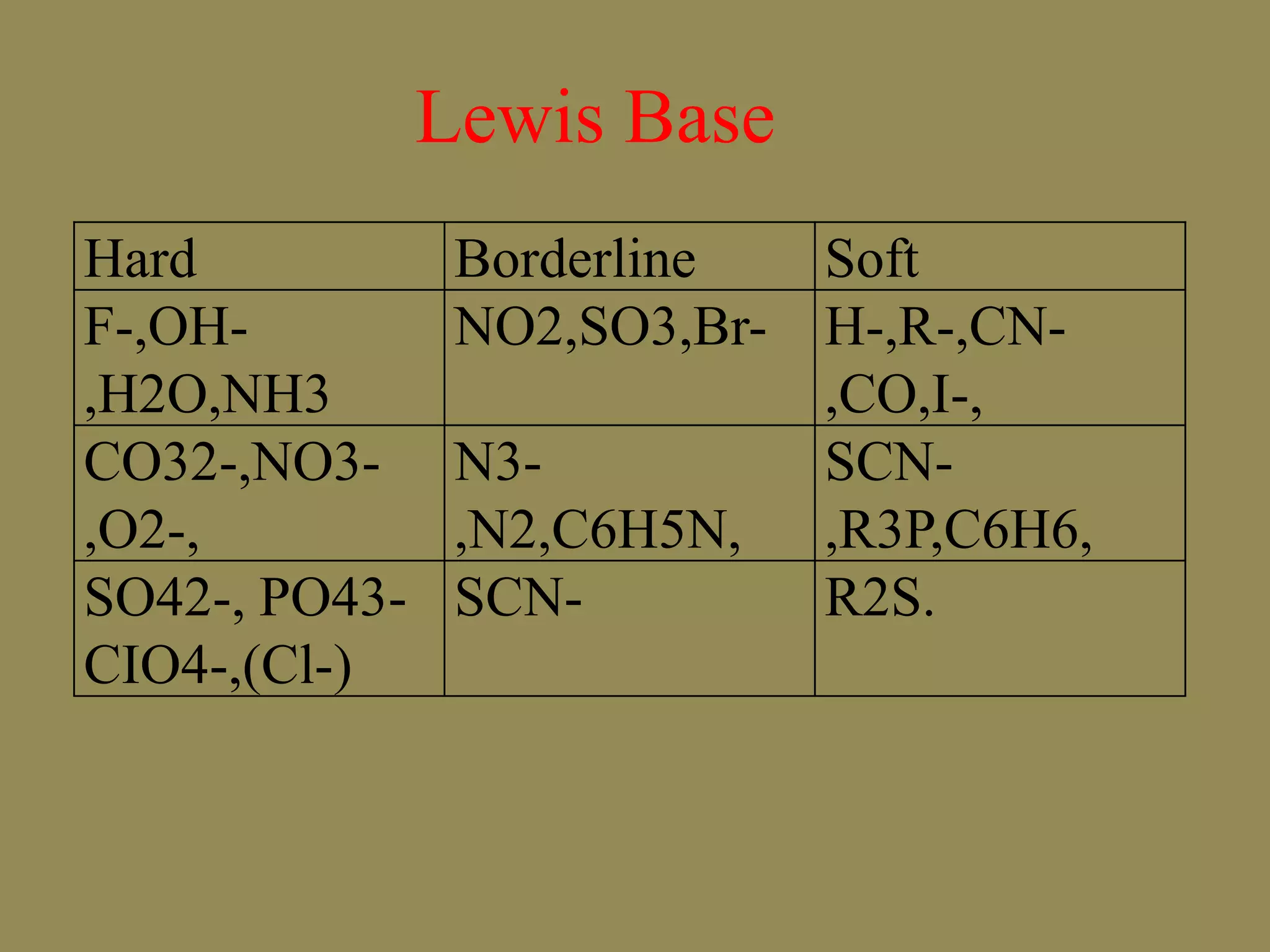
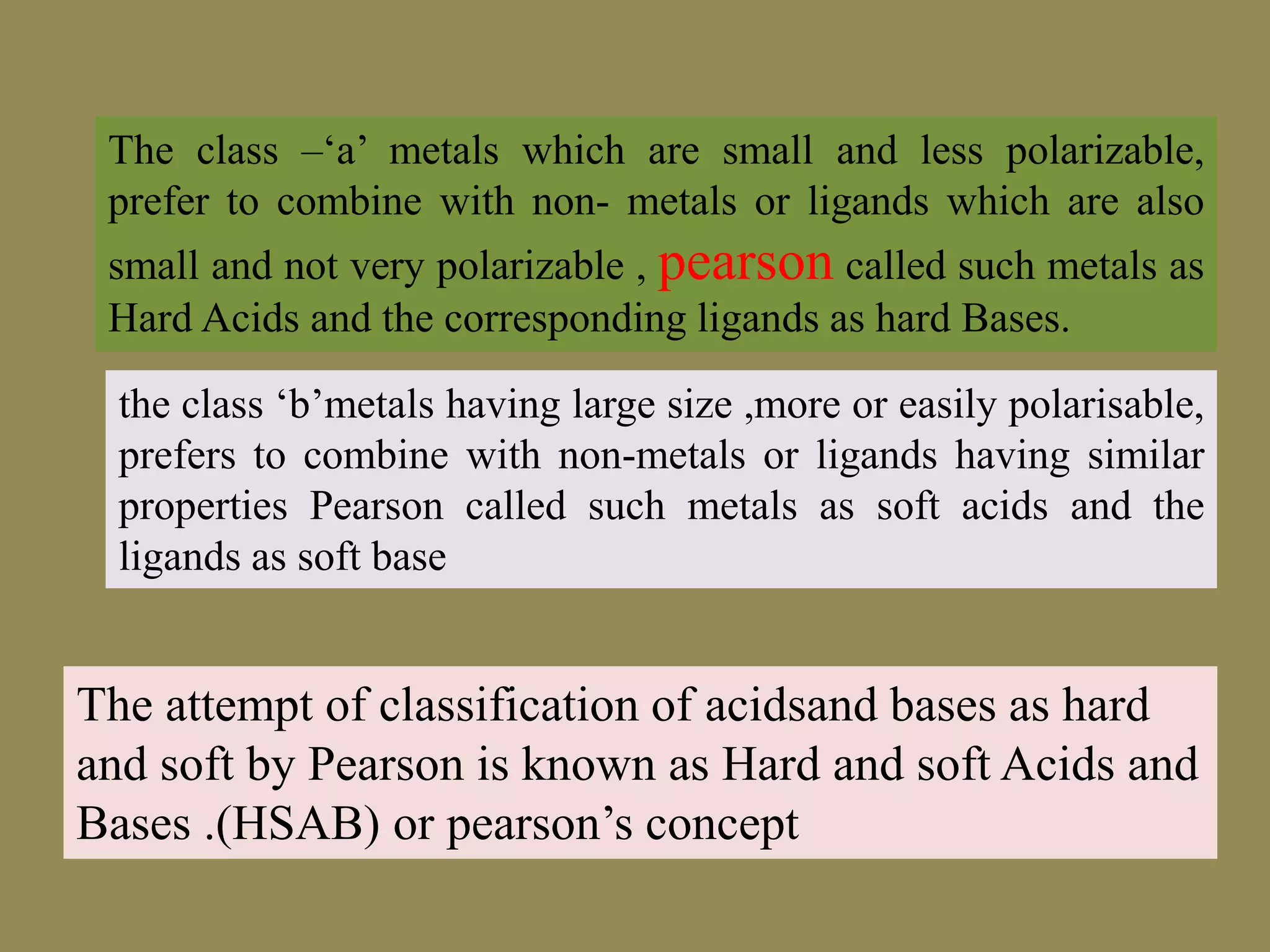
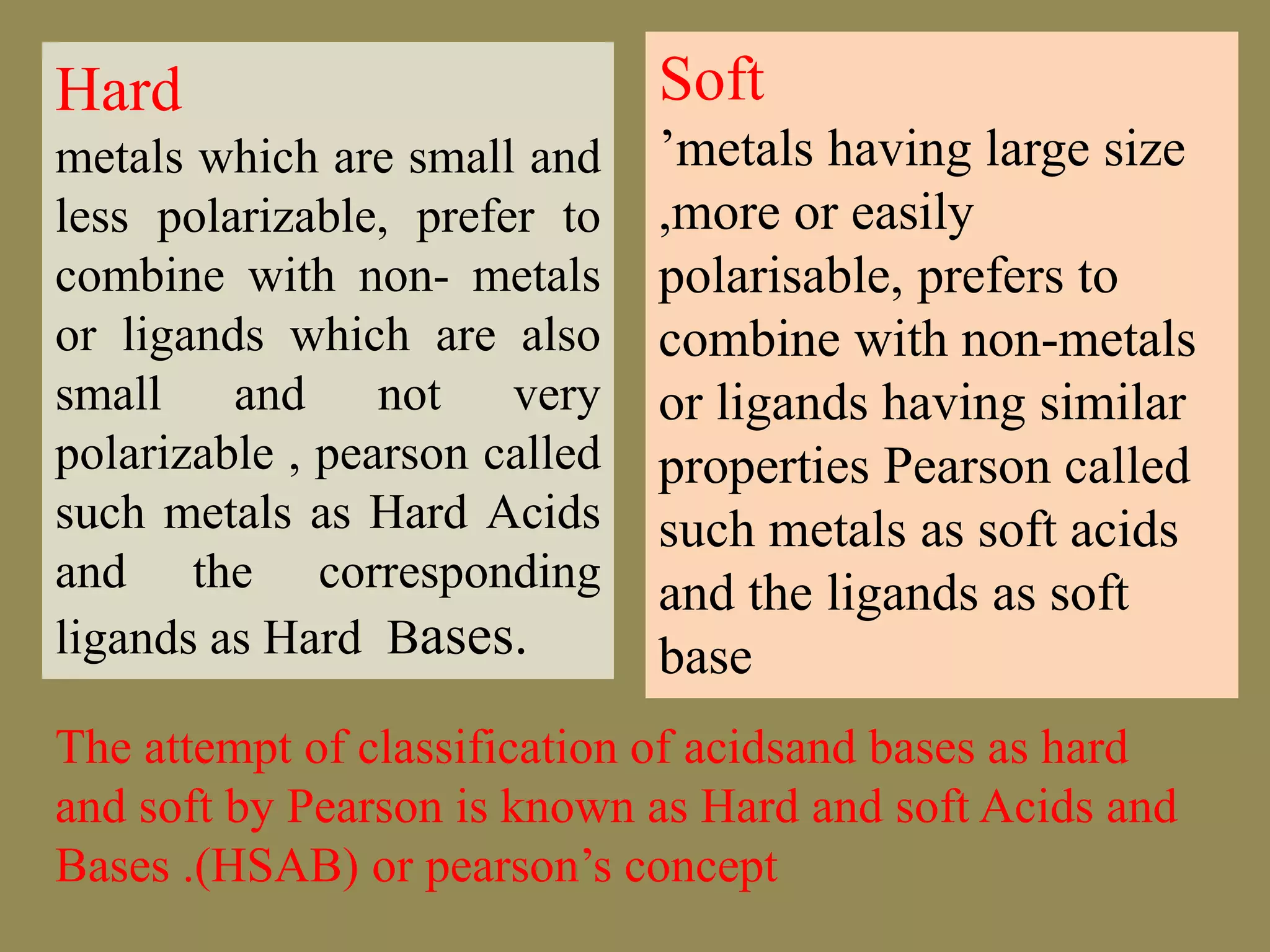
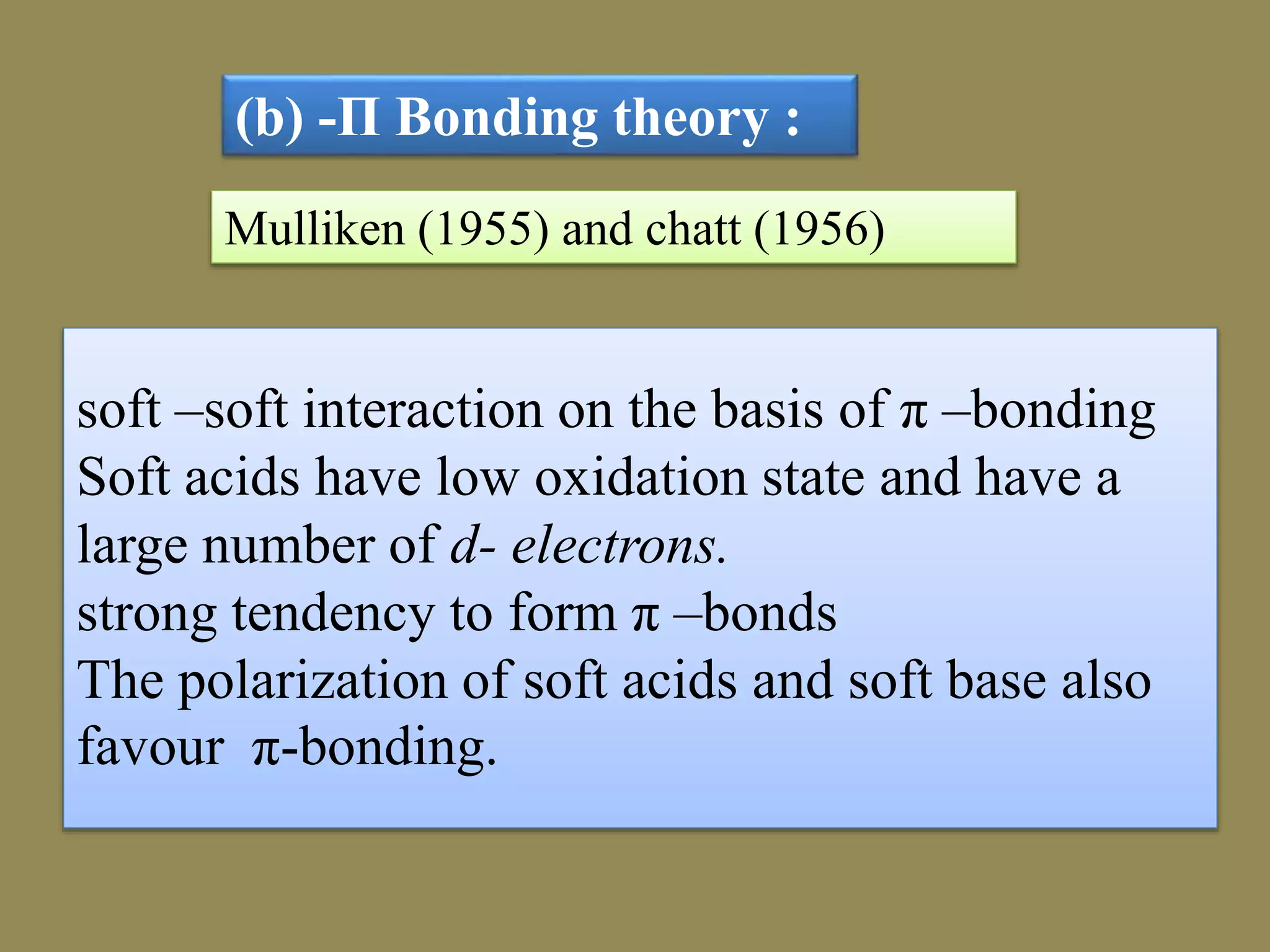
The document discusses hard and soft acid-base (HSAB) theory, which classifies acids and bases as either hard or soft based on their size, charge density, and polarizability. Hard acids and bases are small and nonpolarizable, preferring to interact with each other. Soft acids and bases are larger and more polarizable, preferring to interact with each other. This preferential hard-hard and soft-soft interaction leads to differences in properties like solubility, coordination preferences, and reaction kinetics. The theory has applications in understanding solubility trends, ligand substitution reactions, and the geochemical partitioning of elements.

















![K for ligand exchange reactions
Compare:
[MeHg(H2O)]+ + HCl MeHgCl + H3O+
K= 1.8 x 1012
[MeHg(H2O)]+ + HF MeHgF + H3O+
K= 4.5 x 10-2](https://image.slidesharecdn.com/hard-softacid-basetheory-200819163826/75/Hard-soft-acid-base-theory-23-2048.jpg)