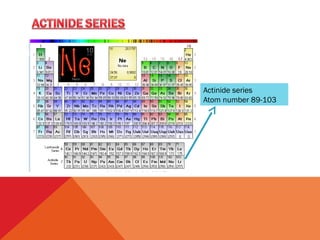
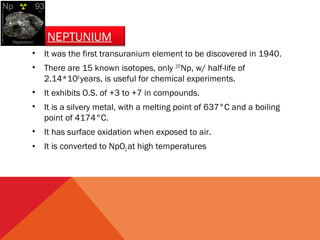
The document discusses the actinide series of elements in the periodic table. It covers the properties and uses of actinium, thorium, protactinium, uranium, neptunium, plutonium, americium, and later actinides like curium. The actinide series includes radioactive elements with atomic numbers from 89 to 103. They have similar chemical properties and most exhibit oxidation states of +3 and +4. Many actinides are used as nuclear fuel or in specialized detection devices.