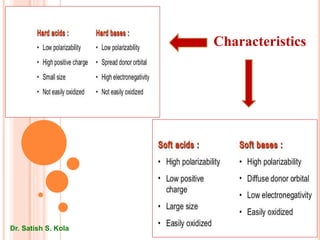
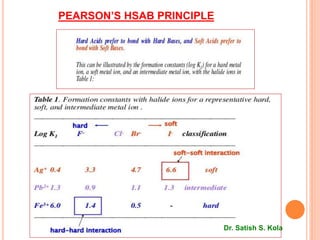
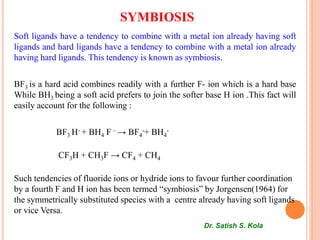
This document discusses Hard and Soft Acids and Bases (HSAB) theory presented by Dr. Satish S. Kola. It defines characteristics of hard vs soft acids and bases, with hard acids/bases being small with high oxidation states and no d-electrons, while soft acids/bases are large with low oxidation states and many d-electrons. Applications of HSAB principles are discussed, including predicting complex formation and metal catalyst poisoning. The theoretical basis involves concepts like pi-bonding, electrostatic interactions, and polarizability. Limitations are noted where inherent acid/base strength may override HSAB predictions.