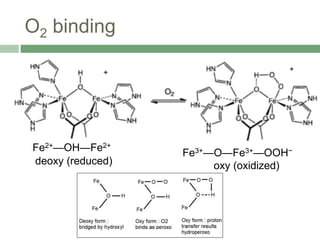
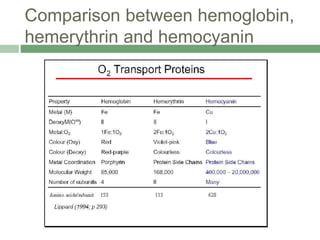
The document discusses non-heme oxygen carrier proteins, specifically hemocyanin and hemerythrin, detailing their structures, mechanisms, and differences from hemoglobin. Hemocyanin, found in invertebrates, contains copper and has a distinct color change during oxygen binding, while hemerythrin, found in some marine invertebrates, contains iron and changes color upon oxygenation. Both proteins are vital for oxygen transport but differ significantly in their efficiency and binding properties.


![Active site of deoxyhemocyanin
Each monomer contains two cuprous ions
[Cu(I)] that reversibly bind one dioxygen.
An empty cavity is present between the
two cuprous ions to accommodate the
dioxygen.
The Cu(I)- Cu(I) bond distance is 460 pm
(no direct interaction between them).
The coordination number of each Cu(I) is
three and is satisfied by three histidines
residues from the protein.
This results in a distorted trigonal
pyramidal geometry.
Two phenylalanine residues which are in
close proximity to the histidines residues
provide a hydrophobic environment at the](https://image.slidesharecdn.com/hemocyaninandhemerythrin-210628102628/85/Hemocyanin-and-Hemerythrin-4-320.jpg)
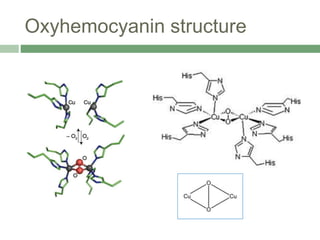
![Active site structure of
oxyhemocyanin
Coordination number of copper changes to five from three.
Geometry of copper changes to square pyramidal from
trigonal pyramidal. The equatorial plane has two histidyl
imidazole nitrogens, the bound oxygens and the third histidyl
nitrogen is axially coordinated to copper.
The Cu-Cu distance decreases to 360 pm.
To accommodate the binding of O2, the protein adjusts its
conformation to bring the two Cu atoms closer together.
The O2-binding site is formulated as Cu(II)-[O2]2--Cu(II)
Coordination of O2 occurs between the two Cu atoms in a
bridging dihapto manner(μ-2-2)](https://image.slidesharecdn.com/hemocyaninandhemerythrin-210628102628/85/Hemocyanin-and-Hemerythrin-6-320.jpg)
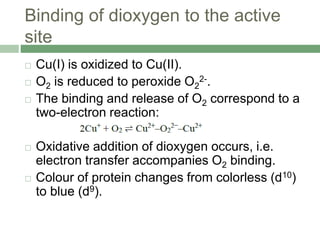
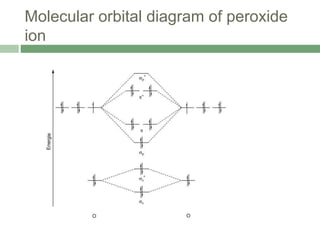
![Experimental observation
Evidence for the peroxo linkage comes from Raman
spectroscopy.
The (O-O) stretching frequency is observed at 744 cm-1
confirming the presence of peroxo linkage
Lowering of the bond order from 2 to 1.
The O2 unit is bound in a bridging mode with an O-O
bond length of 140 pm, typical of that found in peroxide
complexes.
Coordination of dioxygen to Cu(II) is symmetrical
The Cu(II) centres are strongly antiferromagnetically
coupled, with the -[O2]2- ligand being involved in a
superexchange mechanism](https://image.slidesharecdn.com/hemocyaninandhemerythrin-210628102628/85/Hemocyanin-and-Hemerythrin-8-320.jpg)



![Active site structure of
deoxyhemerythrin
Each monomeric unit contains an active site which has two
high spin ferrous ions [Fe(II)].
The ferrous ions are bridged together by a hydroxyl group
and two carboxyl groups from an aspartate residue and a
glutamate residue of the protein chain.
One of the ferrous is hexacoordinated with an octahedral
geometry and the other is pentacoordinated with a distorted
trigonal bipyramidal geometry.
The remaining coordination sites of hexacoordinated ferrous
and pentacoordinated ferrous are satisfied by three and two
imidazole nitrogens respectively from histidine residues of the
protein chain
The hydroxyl group serves as a bridging ligand but also
functions as a proton donor to the O2 substrate.](https://image.slidesharecdn.com/hemocyaninandhemerythrin-210628102628/85/Hemocyanin-and-Hemerythrin-14-320.jpg)