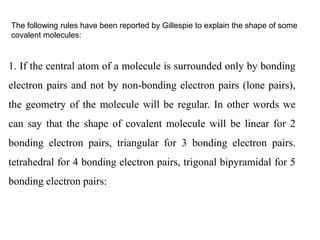
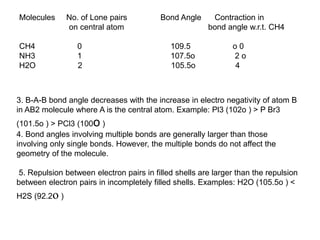
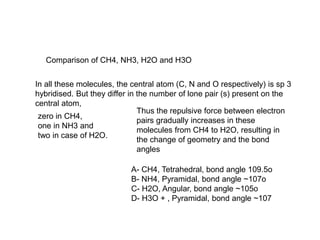
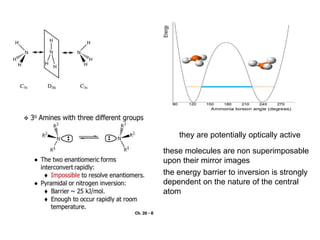
This document summarizes several theories and concepts related to stereochemistry in main group compounds, including:
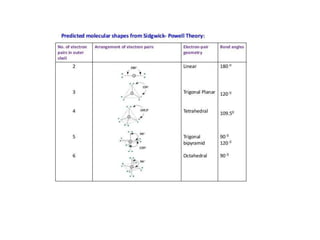
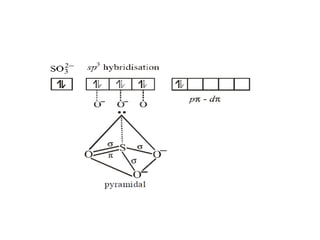
1) VSEPR theory which describes the geometry of molecules based on electron pairs around the central atom. It explains linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral geometries.
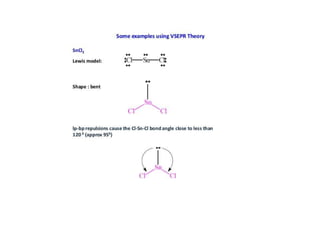
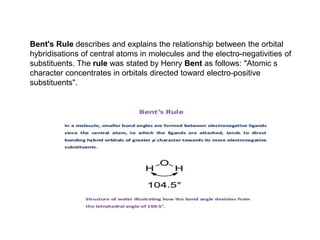
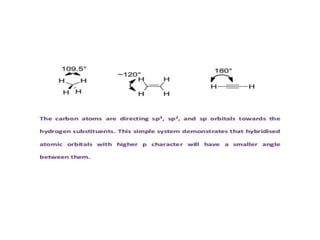
2) Bent's rule which describes how atomic s-character concentrates in orbitals directed toward electropositive substituents.
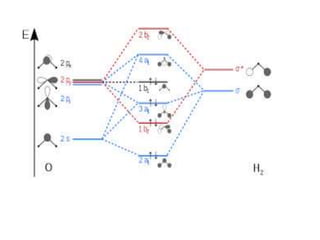
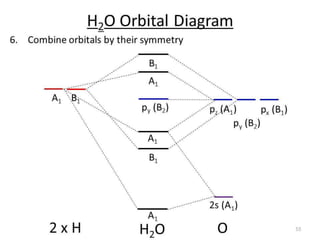
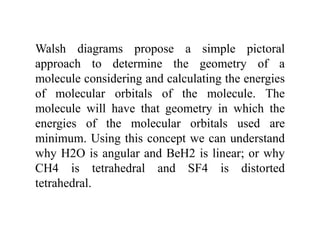
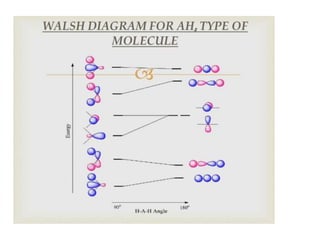
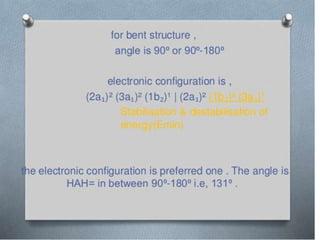
3) Walsh diagrams which use molecular orbital energies to determine molecular geometry.
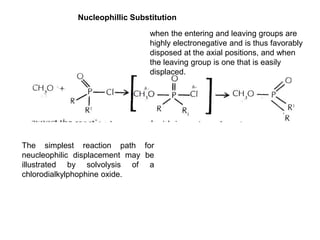
4) Berry pseudorotation and fluxionality concepts which explain rapid ligand exchange in molecules like PF5.






![Comparison of PF5, SF4, ClF3 and [ICl2 - ]
a) PF5, Trigonal bipyamidal
b) (b) SF4, Irregular tetrahedral
c) (c) ClF3, T-Shaped
d) (d) [ICl2] - , Linear](https://image.slidesharecdn.com/stereochemistryinmaingroupcompounds-210126001505/85/Stereochemistry-in-main-group-compounds-11-320.jpg)

















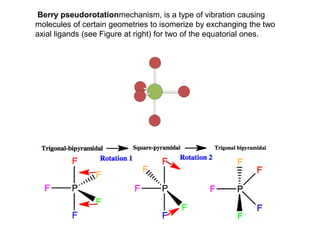
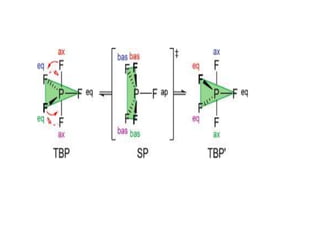
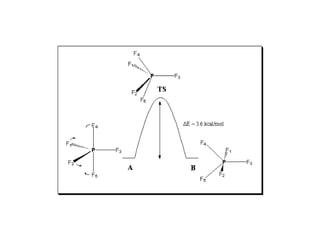
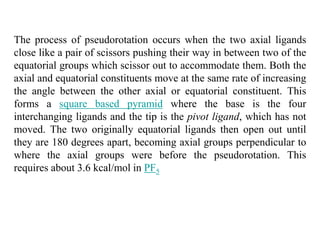
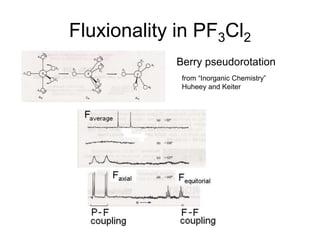
![Single-crystal X-ray studies indicate that the PF5 has trigonal bipyramidal
geometry. Thus it has two distinct types of P−F bonds (axial and
equatorial): the length of an axial P−F bond is distinct from the equatorial
P-F bond in the solid phase, but not the liquid or gas phases due to
Pseudo Berry Rotation.
Fluorine-19 NMR spectroscopy, even at temperatures as low as −100 °C,
fails to distinguish the axial from the equatorial fluorine environments. The
apparent equivalency arises from the low barrier for pseudorotation via
the Berry mechanism, by which the axial and equatorial fluorine atoms
rapidly exchange positions. The apparent equivalency of the F centers in
PF5 was first noted by Gutowsky.[3]The explanation was first described
by R. Stephen Berry, after whom the Berry mechanism is named. Berry
pseudorotation influences the 19F NMR spectrum of PF5 since NMR
spectroscopy operates on a millisecond timescale. Electron diffraction and
X-ray crystallography do not detect this effect as the solid state structures
are, relative to a molecule in solution, static and can not undergo the
necessary changes in atomic position.](https://image.slidesharecdn.com/stereochemistryinmaingroupcompounds-210126001505/85/Stereochemistry-in-main-group-compounds-50-320.jpg)