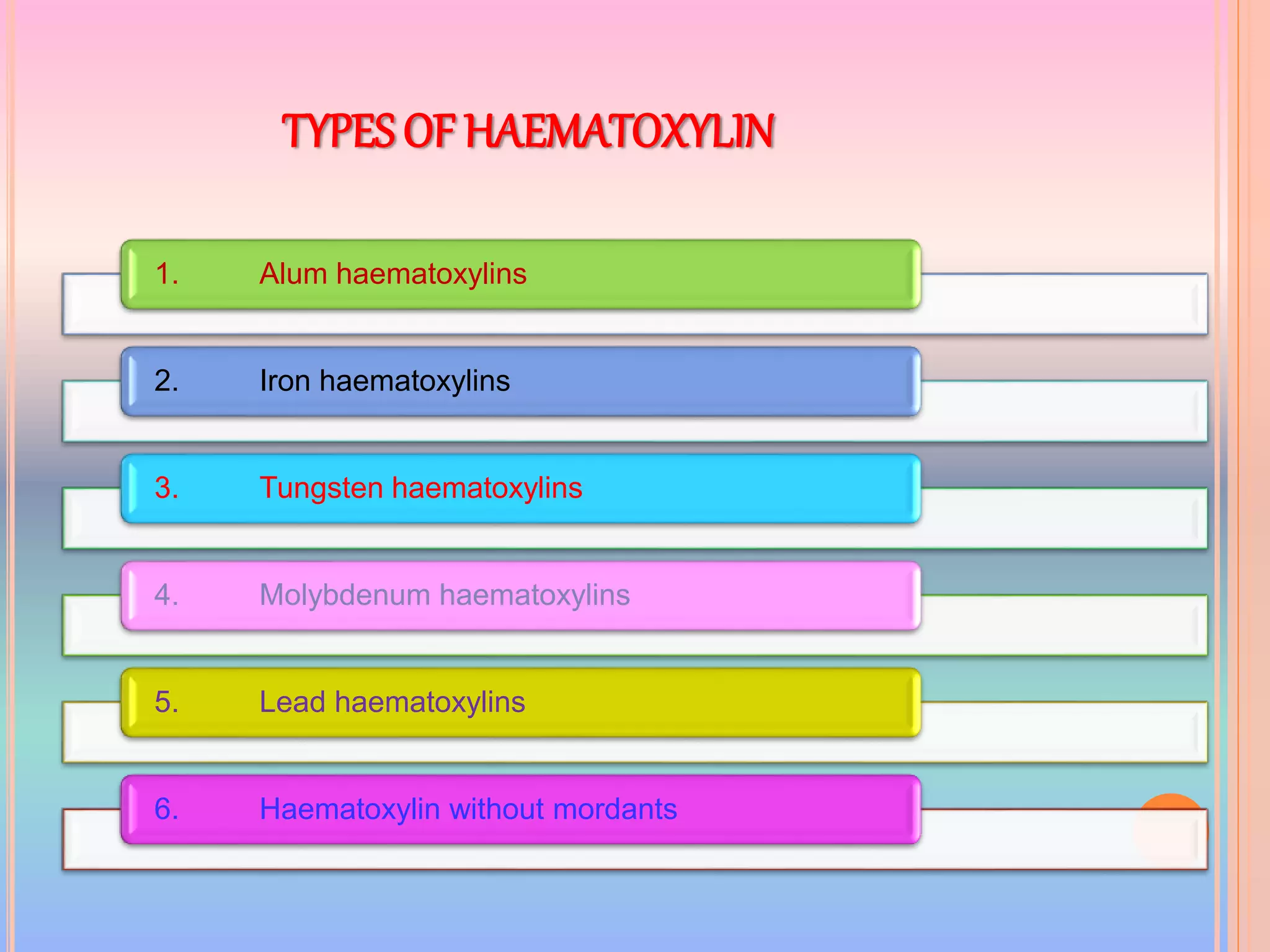
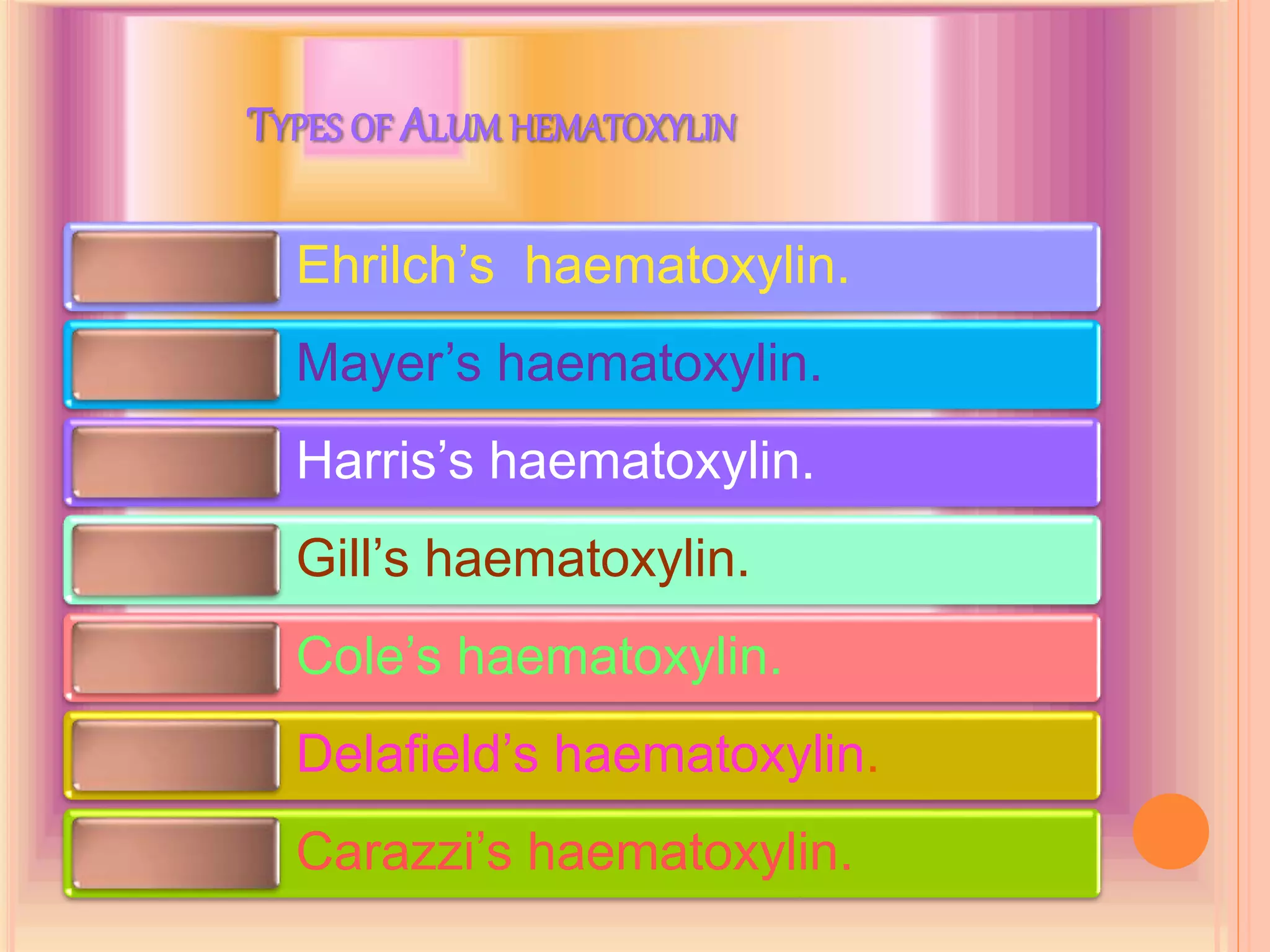
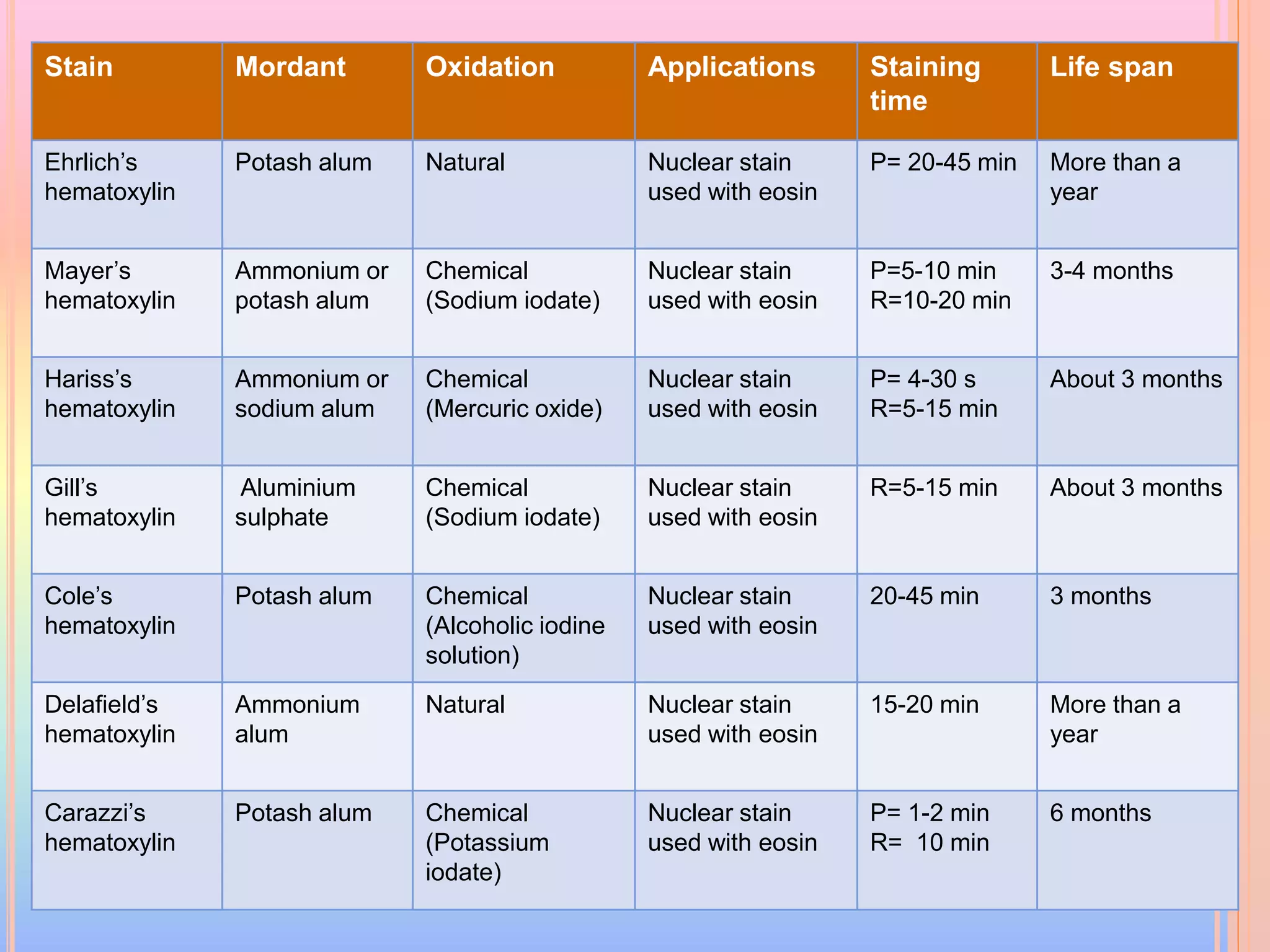
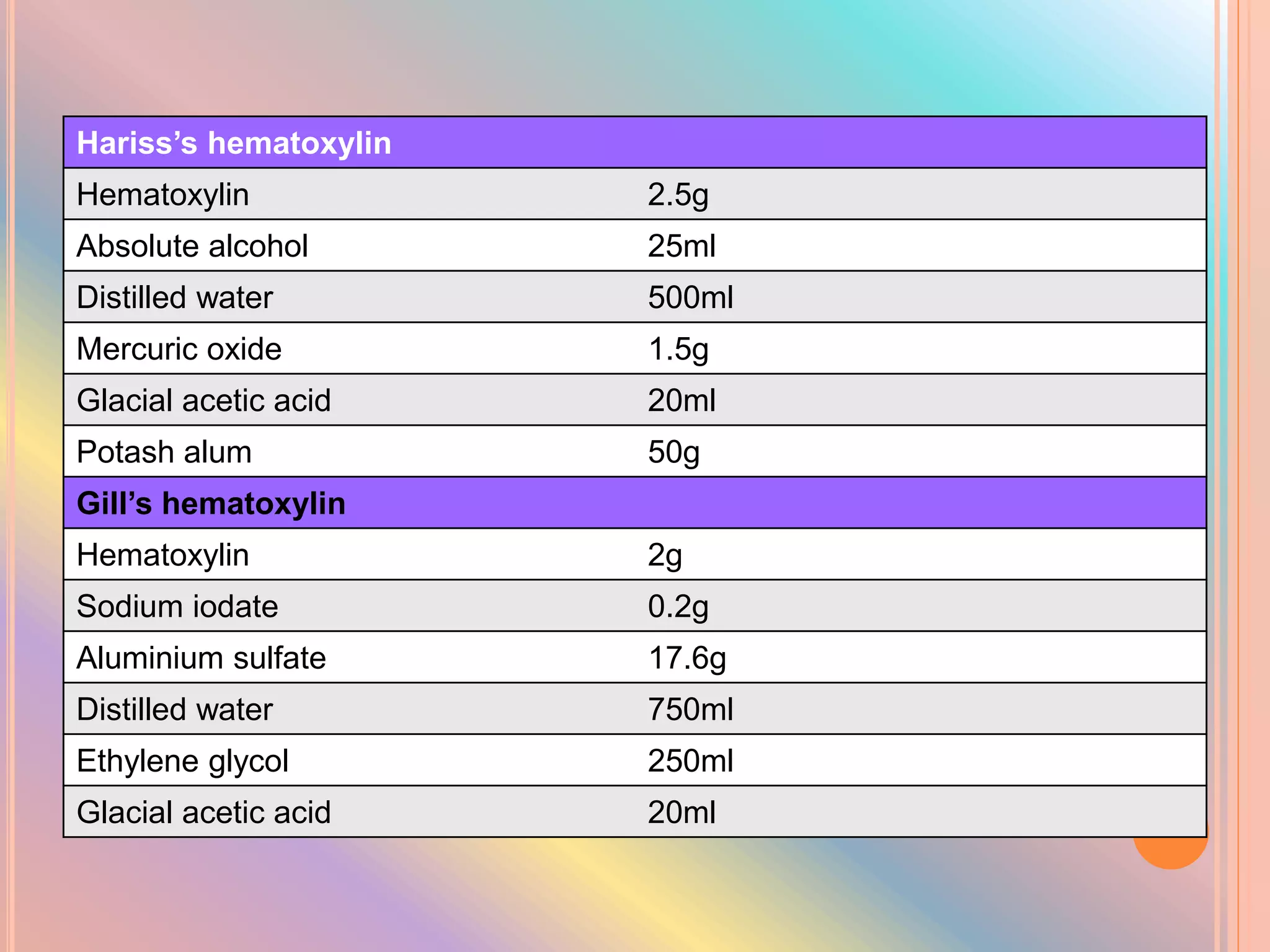
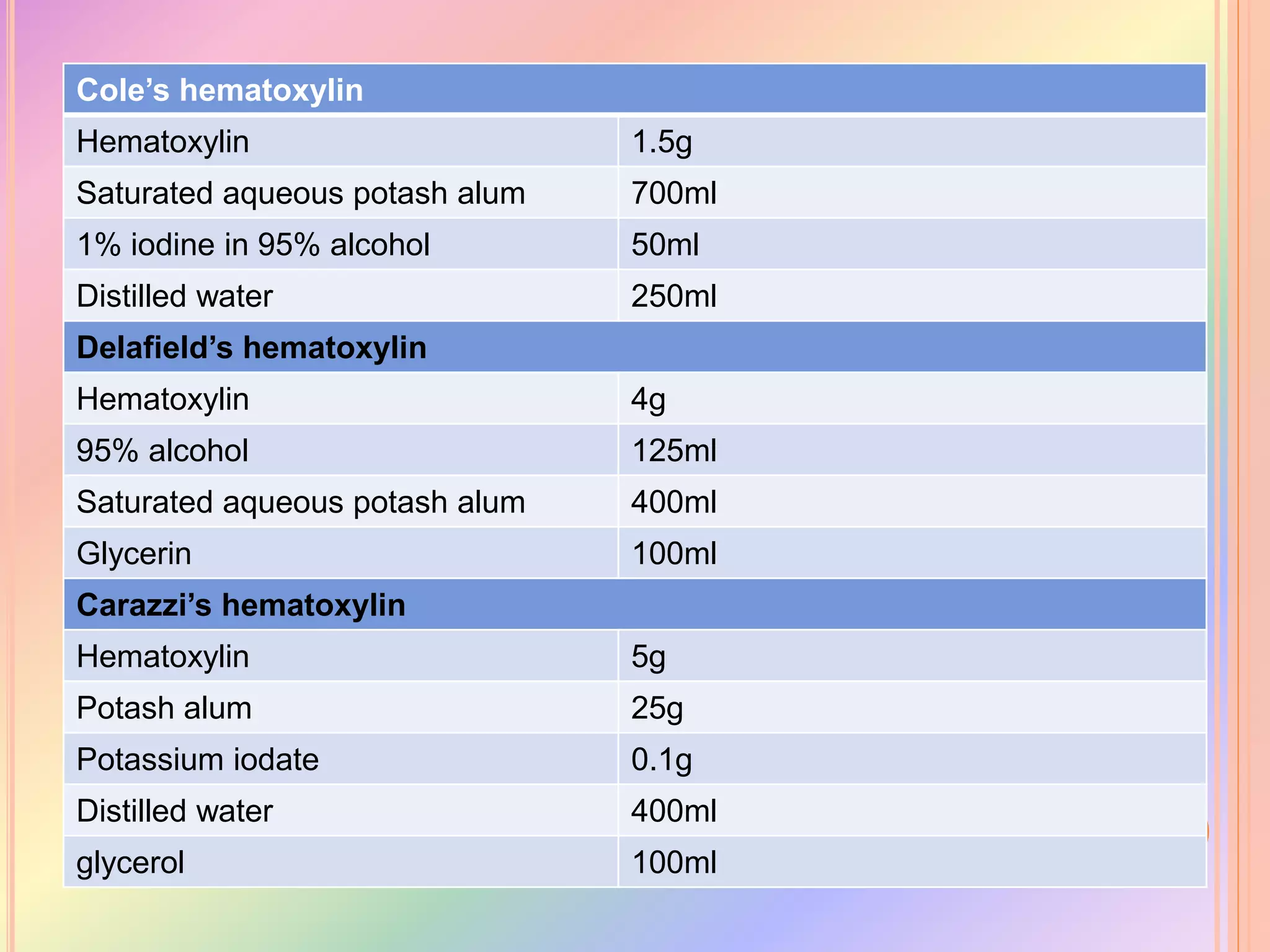
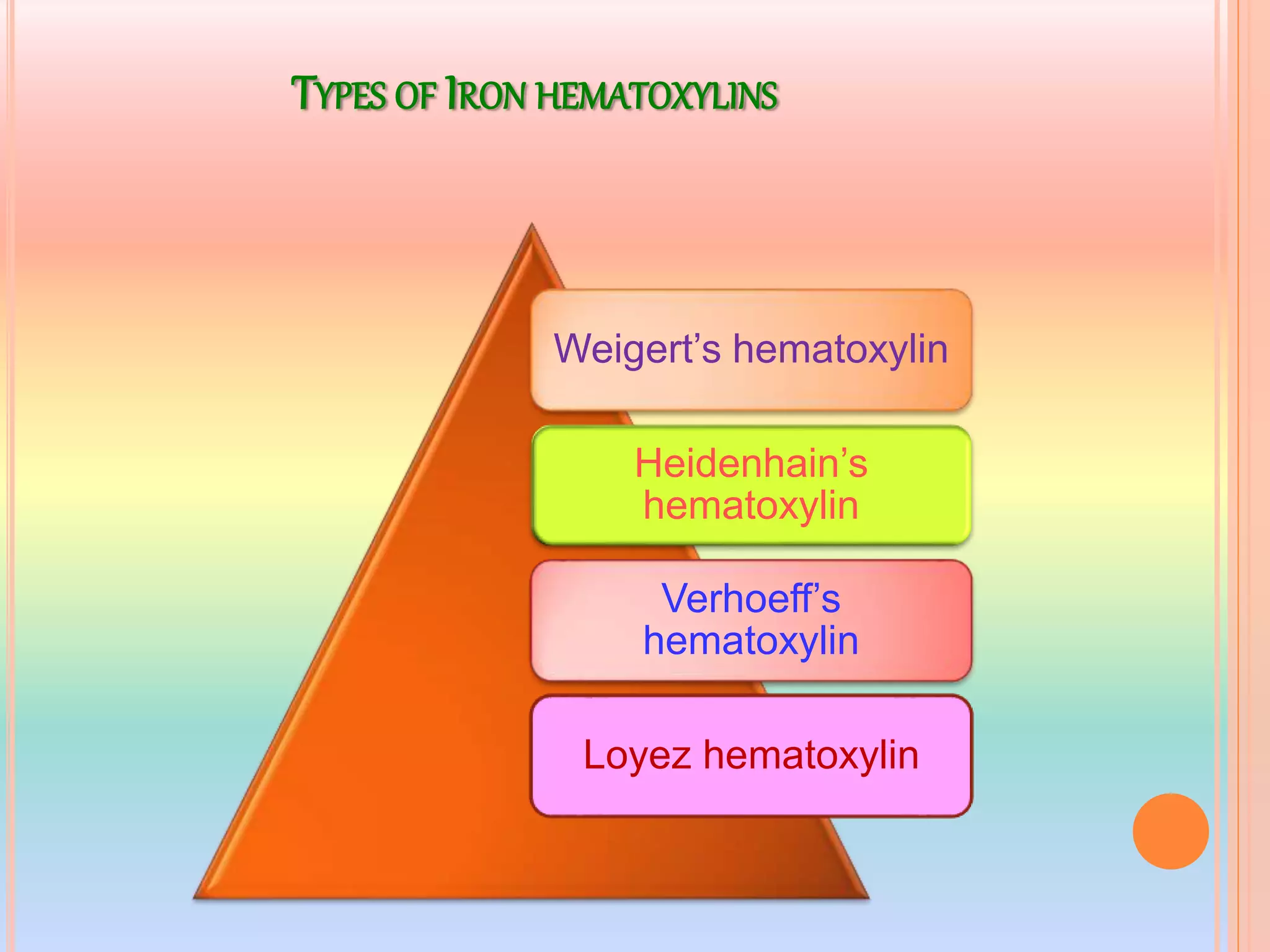
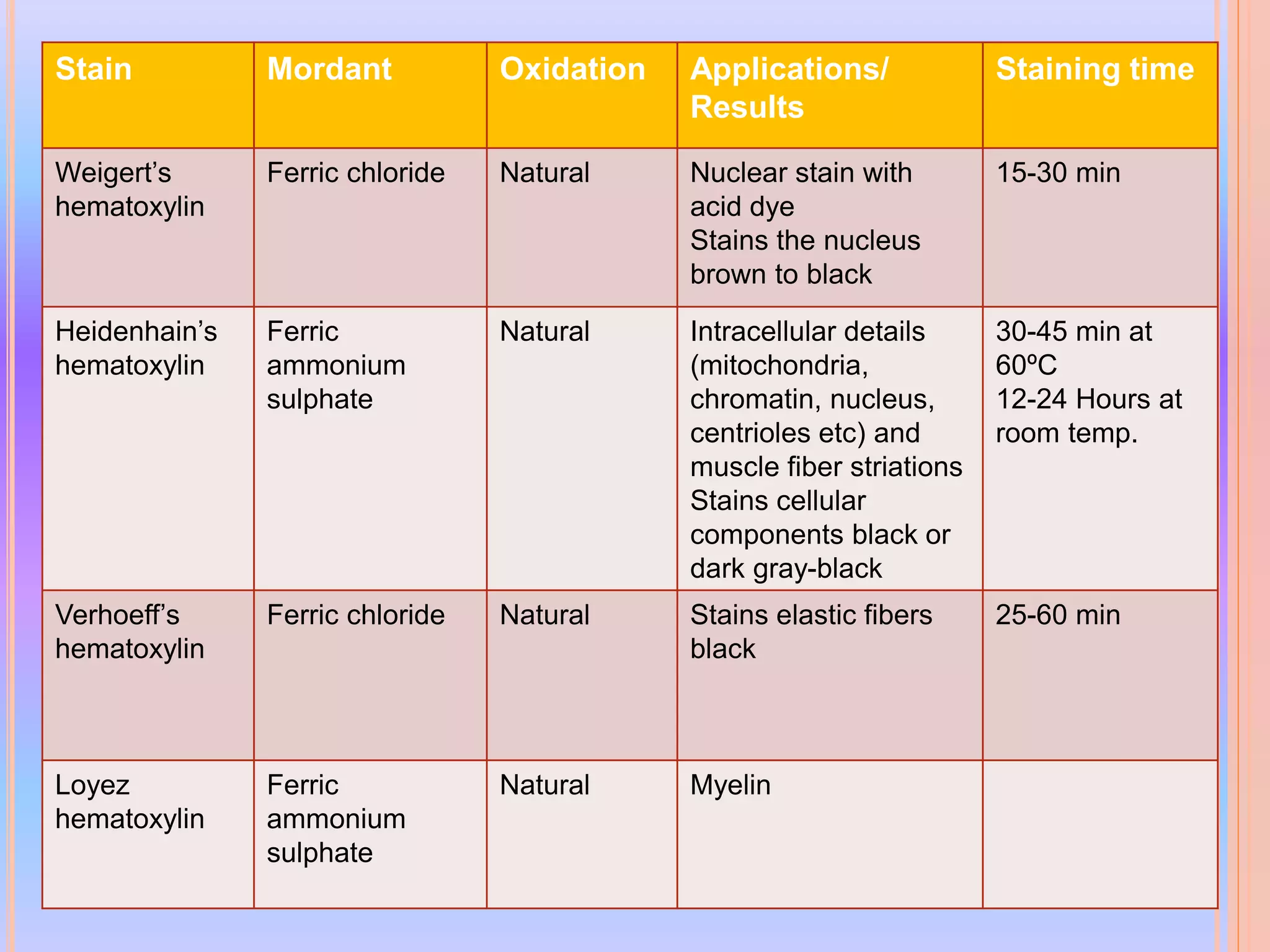
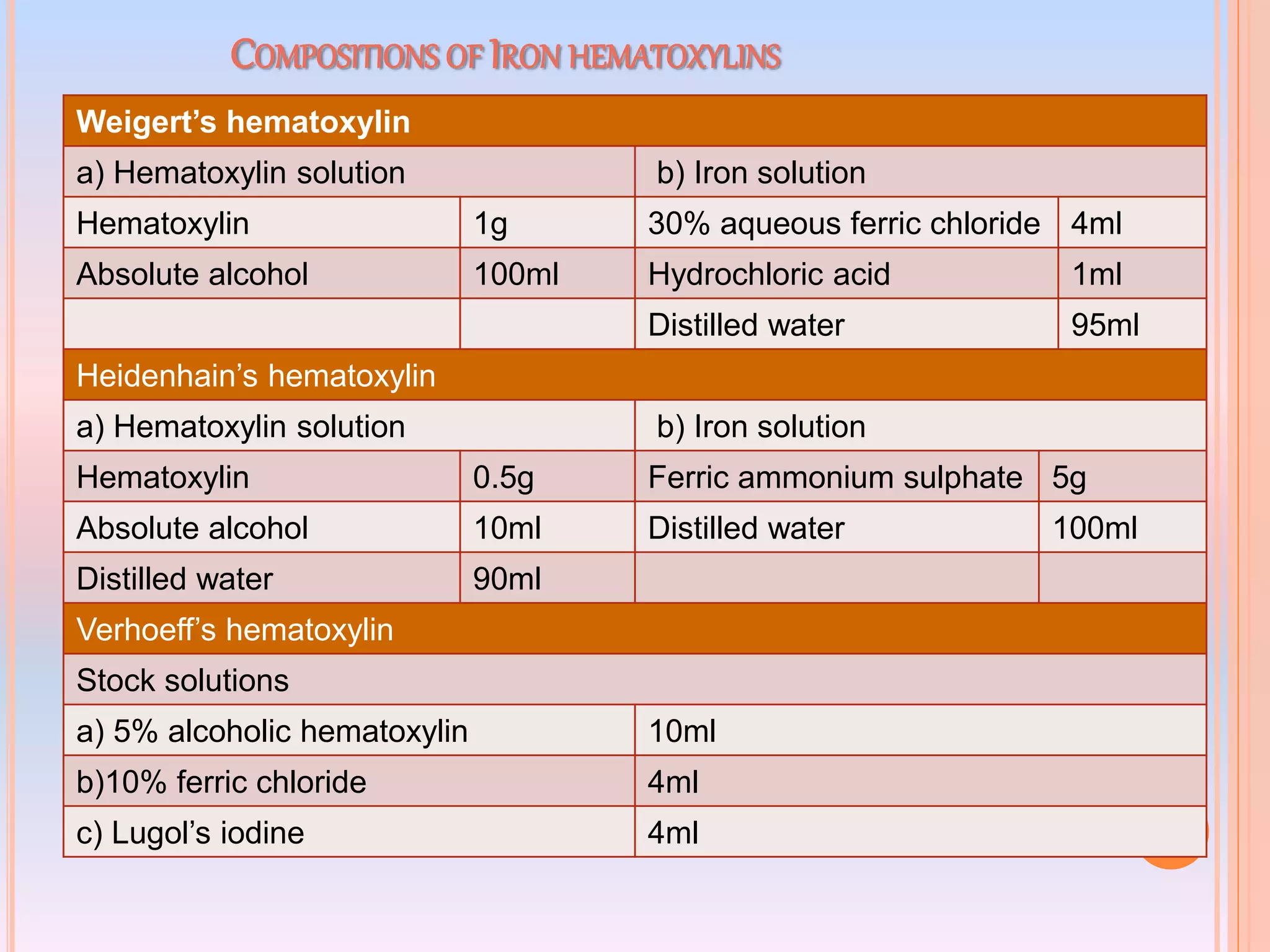
Hematoxylin is a natural dye derived from the log wood of Haematoxylon campechianum, widely used in histopathology and cytology for staining tissues, particularly nuclei. It requires a mordant for effective staining, which can be achieved through two oxidation methods: natural and chemical, resulting in various types of haematoxylin suited for different applications. The document details various formulations, types, and uses of haematoxylins, including alum, iron, tungsten, and molybdenum haematoxylins, along with their application protocols and compositional specifics.