1. Hematoxylin and eosin staining is the most widely used staining technique in histopathology. Hematoxylin stains nuclei blue/black while eosin stains cytoplasm and extracellular components pink.
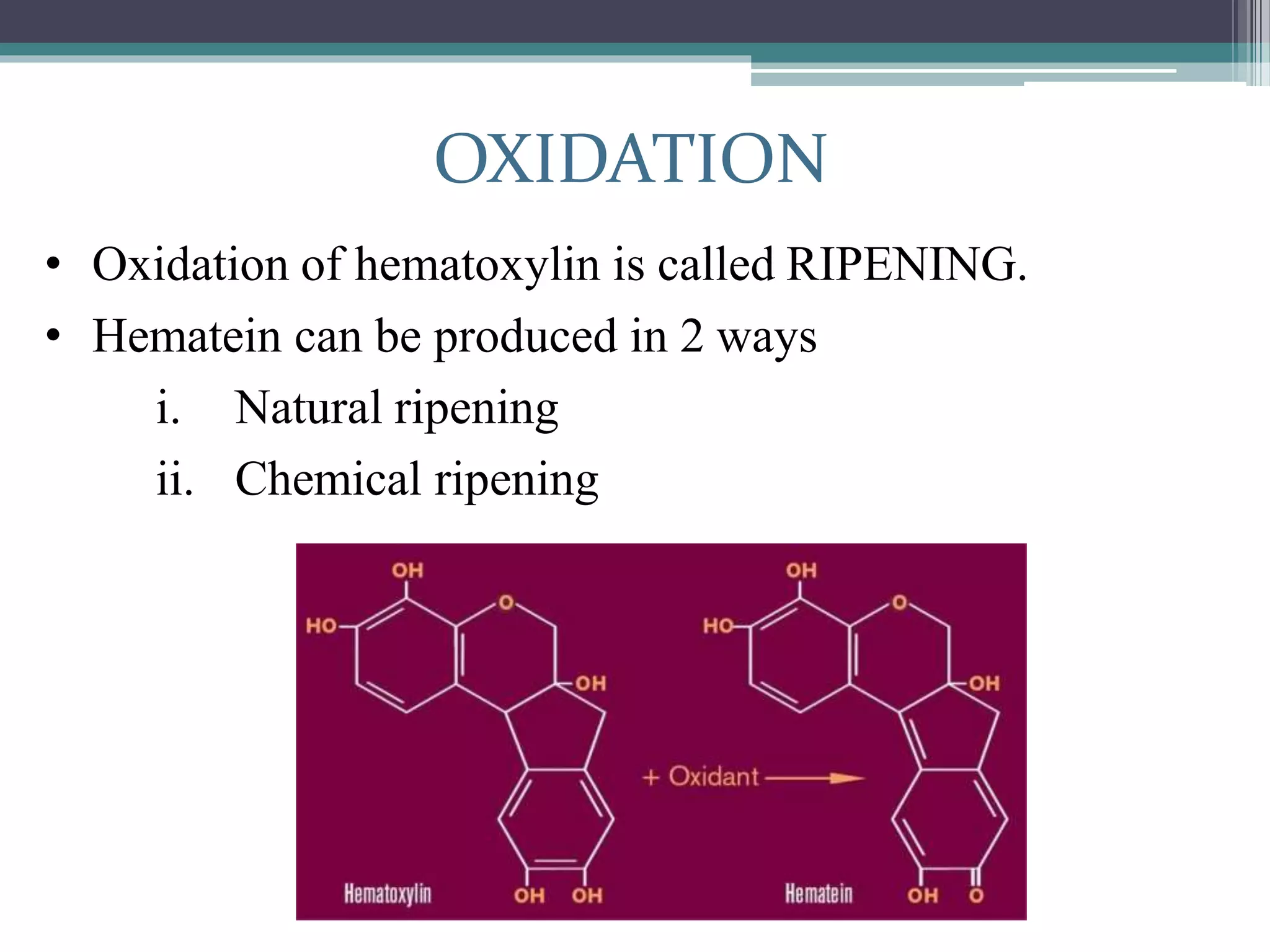
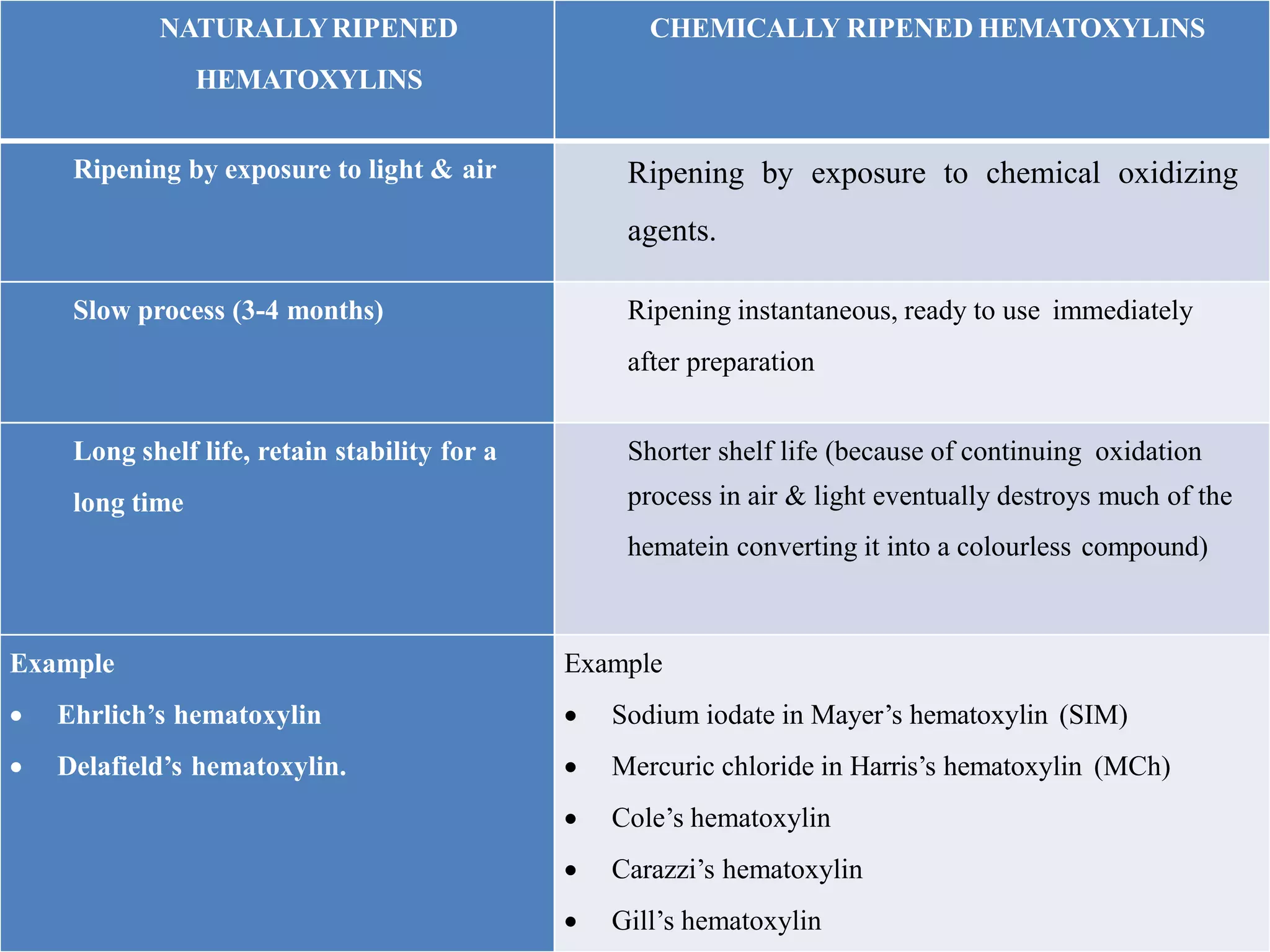
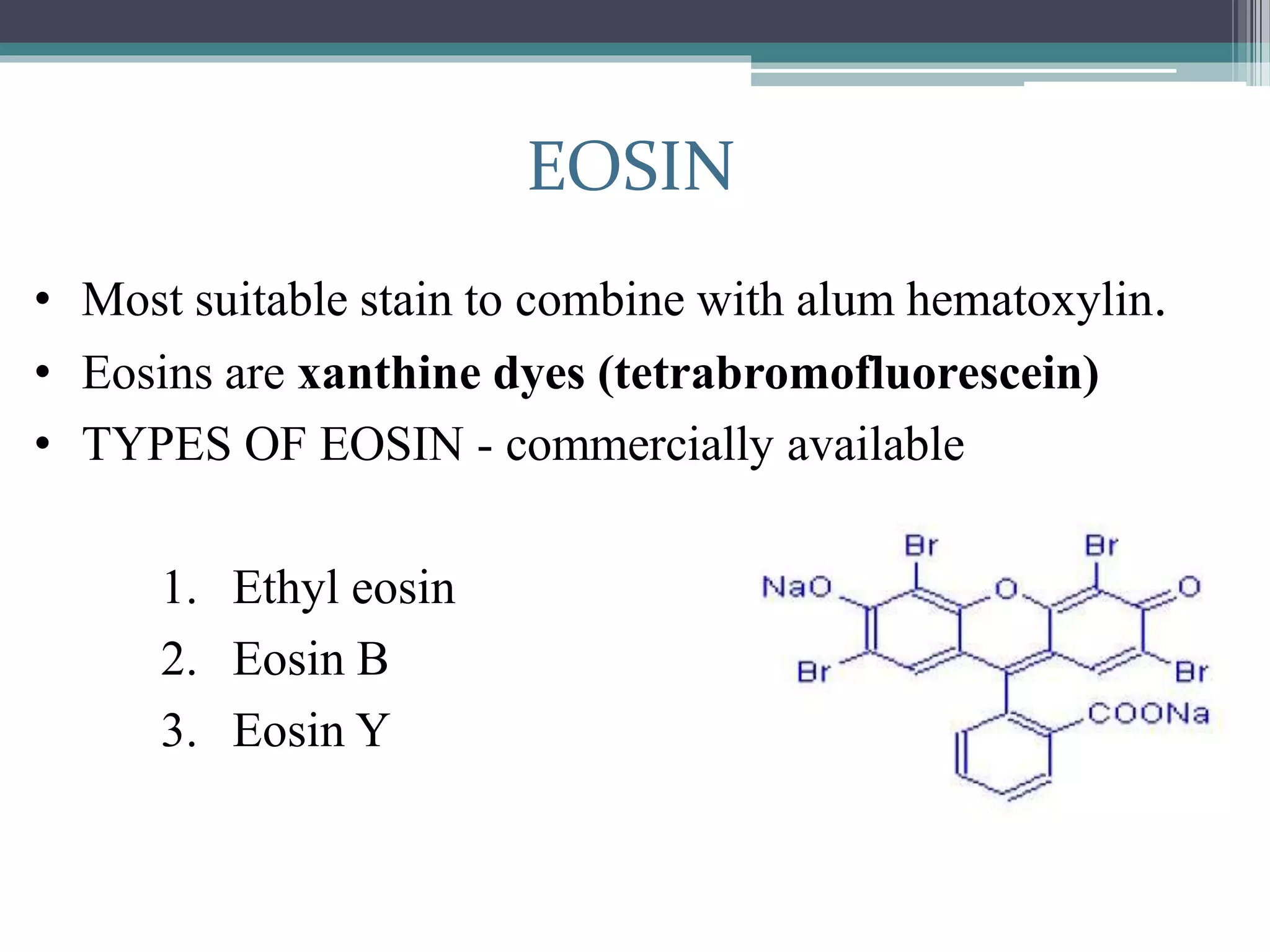
2. Hematoxylin requires oxidation to produce hematein, the active dye, and uses a mordant like aluminum or iron salts to bind it to tissue. Eosin is a xanthine dye that stains cytoplasm and extracellular components red.
3. The basic steps of hematoxylin and eosin staining involve staining with hematoxylin, differentiating, bluing, staining with eosin, dehydration and mounting. Proper timing is needed for each step to achieve optimal