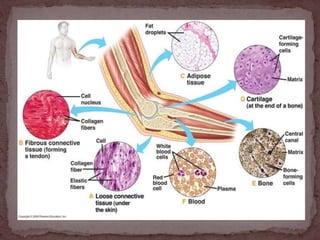
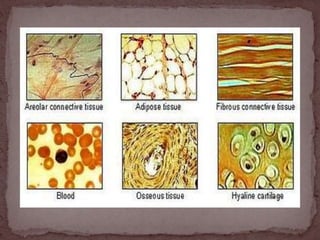
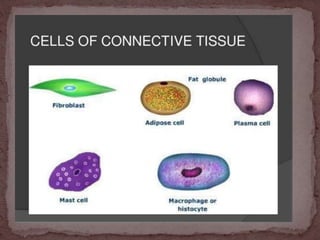
This document provides information on various histological staining techniques used to identify different types of tissues and biomolecules. It discusses connective tissue stains like Van Gieson's stain, Masson's trichrome stain, and Verhoeff's stain used to identify collagen fibers. It also describes reticulin stains, elastic stains, carbohydrate stains like periodic acid Schiff, and mucin stains like Alcian blue to identify different components of tissues under the microscope. Procedures for each stain are outlined along with the expected results.