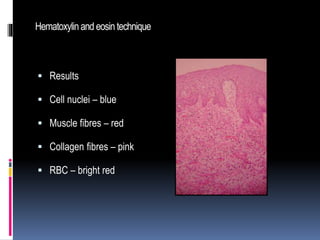
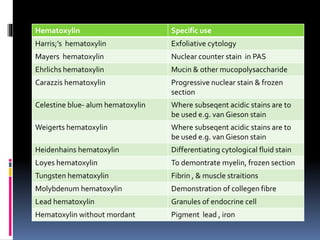
Hematoxylin and eosin staining is a common histological technique that uses hematoxylin, which stains cell nuclei blue, and eosin, which stains cytoplasm and connective tissue pink. The document describes the full H&E staining procedure, including dewaxing, hydration, staining, differentiation, dehydration, clearing and mounting of tissue sections. It also discusses the principles and properties of hematoxylin, including how it is extracted from logwood and requires oxidation or "ripening" to become an effective nuclear stain. Commonly used hematoxylin formulations including Harris's, Mayer's, and Ehrlich's are compared.