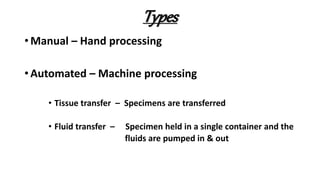
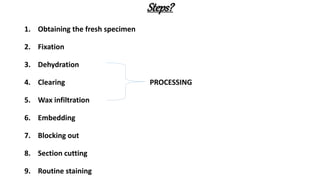
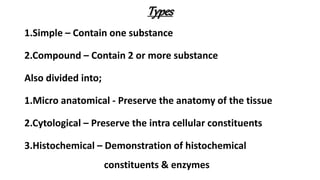
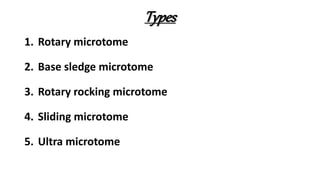
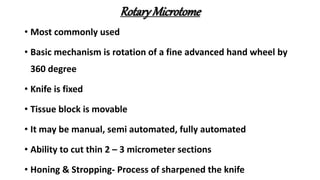
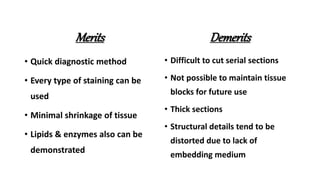
This document describes the steps involved in tissue processing from fixation to embedding in wax. It discusses obtaining fresh specimens, fixation in formalin, dehydration through an alcohol series, clearing in xylene, infiltration and embedding in paraffin wax. Sections are then cut on a microtome, mounted on slides and stained, usually with hematoxylin and eosin, to visualize tissue structures microscopically. Proper processing is important to preserve tissue morphology and produce high quality stained sections for diagnostic examination.