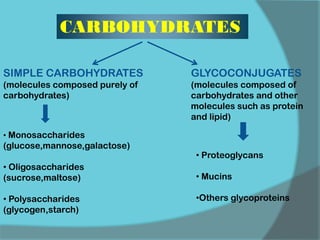
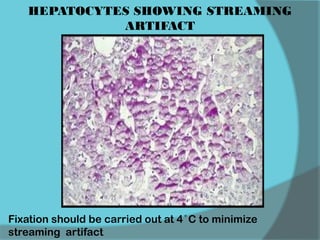
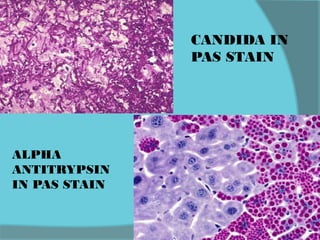
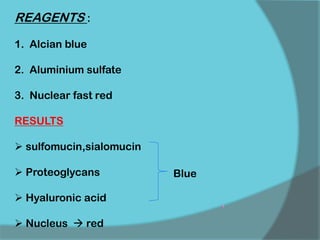
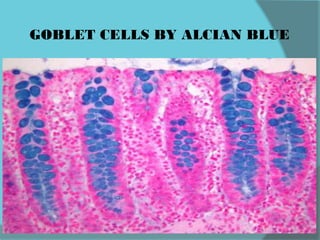
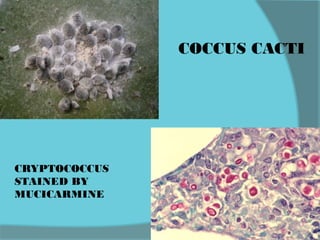
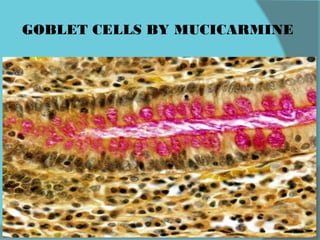
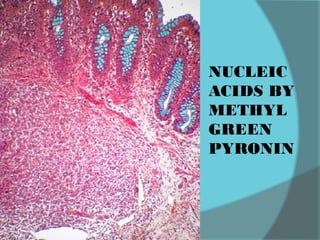
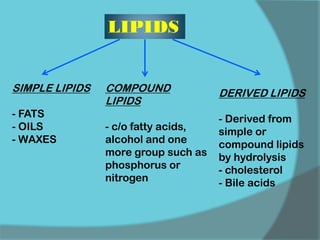
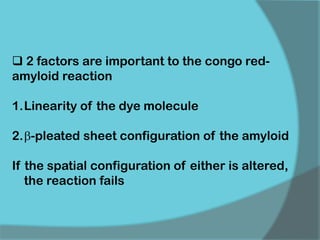
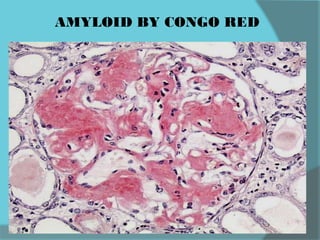
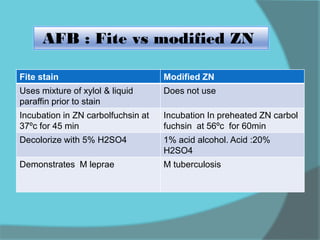
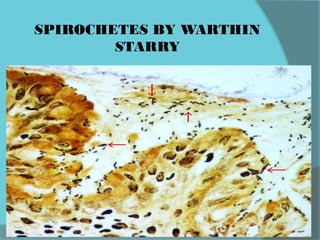
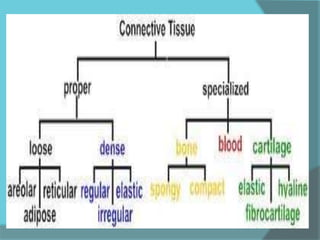
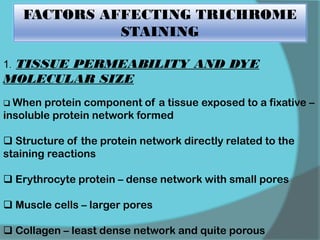
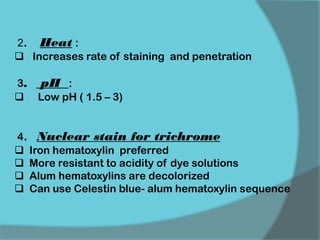
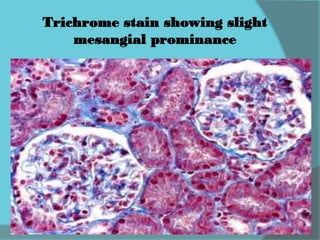
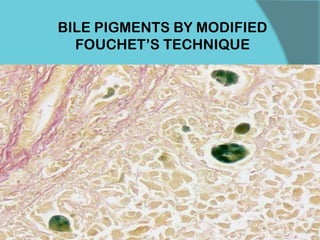
The document discusses various histological staining techniques. It begins by explaining hematoxylin and eosin staining, which provides basic diagnostic information. It then covers special stains that highlight specific tissue components, categorized by the structures they identify such as carbohydrates, amyloid, lipids, nucleic acids, and microorganisms. Carbohydrate stains discussed include periodic acid Schiff, alcian blue, mucicarmine, and others. Amyloid identification using Congo red and methyl violet is explained. Lipid stains using Sudan dyes are also summarized. The document provides details on techniques for staining nucleic acids and identifying bacteria by Gram staining.