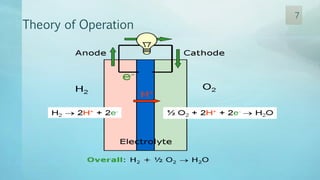
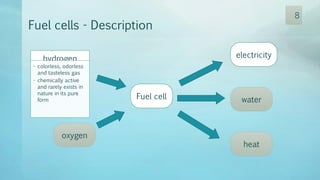
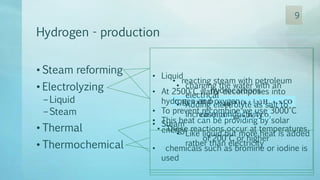
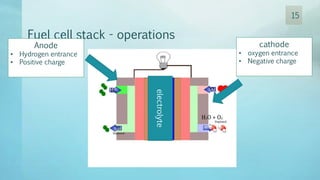
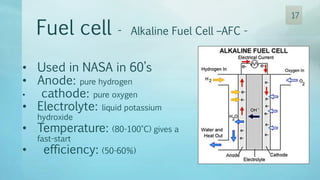
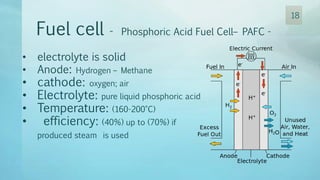
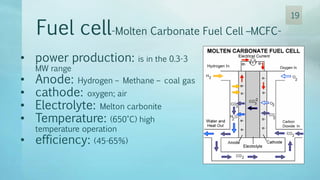
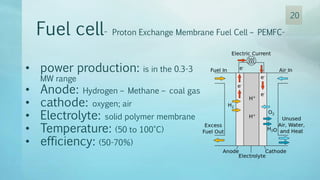
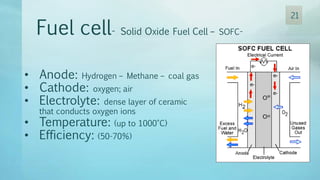
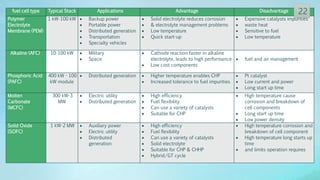
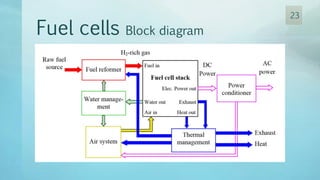
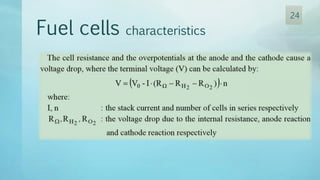
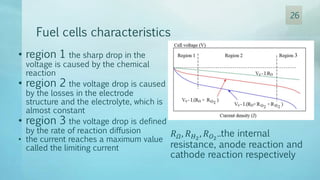
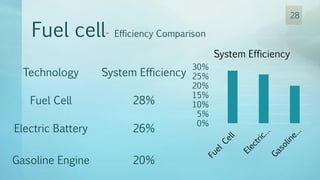
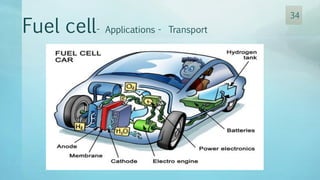
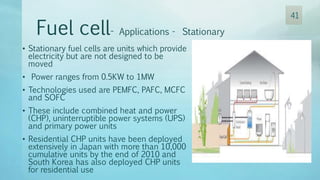
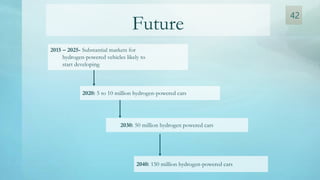
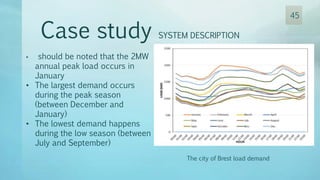
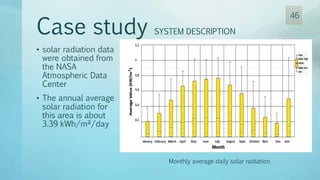
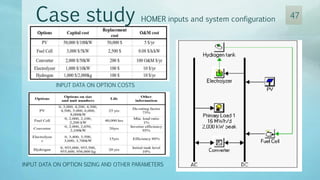
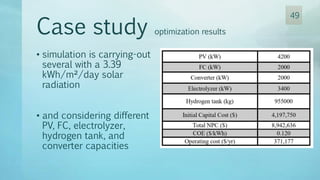
The document discusses hydrogen energy and fuel cells. It provides an introduction and history of fuel cells, explaining their theory of operation. It then discusses hydrogen production and storage methods. The document outlines the different types of fuel cells - alkaline, phosphoric acid, molten carbonate, proton exchange membrane, and solid oxide fuel cells - and compares their characteristics. It also covers fuel cell electrical properties, efficiency comparisons to other technologies, advantages and disadvantages, and applications. The overall document provides a comprehensive overview of hydrogen energy and the different aspects of fuel cells.