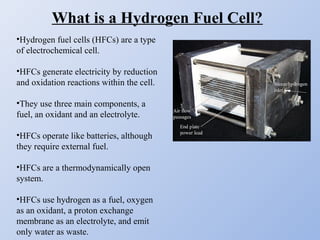
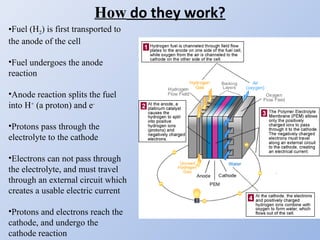
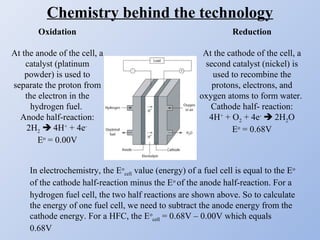
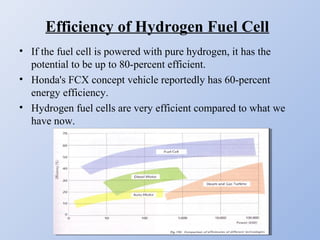
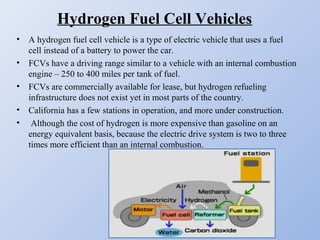
Hydrogen fuel cells generate electricity through an electrochemical reaction of hydrogen and oxygen without combustion. They work like batteries and can provide power as long as they have a source of hydrogen fuel. A hydrogen fuel cell uses hydrogen as fuel, oxygen as an oxidant, a proton exchange membrane as the electrolyte, and produces only water as a byproduct. It has the potential to be more efficient than combustion engines and emits no harmful emissions. However, hydrogen fuel cells still face challenges related to production, storage and transportation of hydrogen as well as the high cost of platinum catalysts.