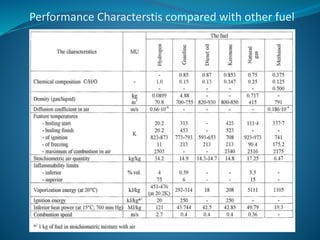
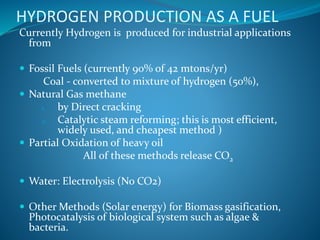
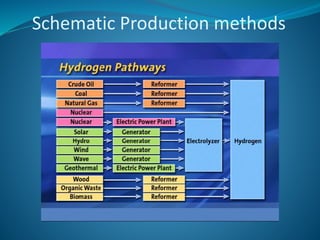
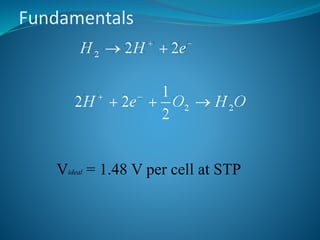
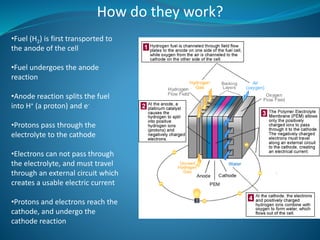
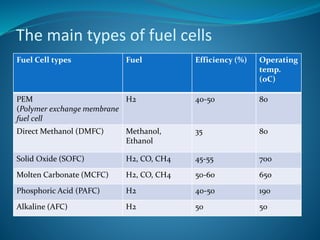
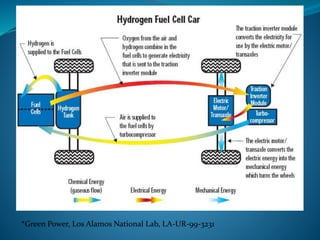
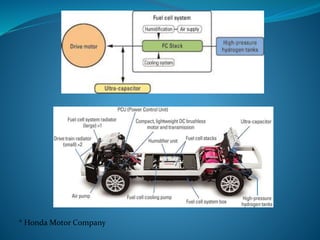
This document discusses hydrogen fuel cells as an alternative fuel for automobiles. It describes how hydrogen has the highest energy content per unit mass of all fuels and can be produced from renewable sources. The document outlines the main properties of hydrogen, compares its performance to other fuels, and lists advantages like being renewable and emitting no CO2, as well as limitations like storage challenges and lack of infrastructure. It also explains how hydrogen fuel cells work to produce electricity from hydrogen and oxygen, and discusses types of fuel cells and possible large-scale applications.