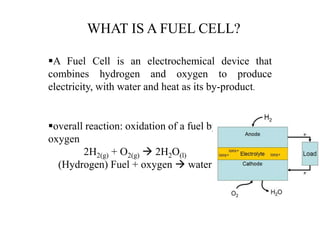
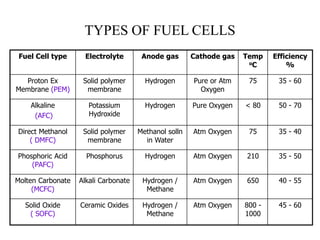
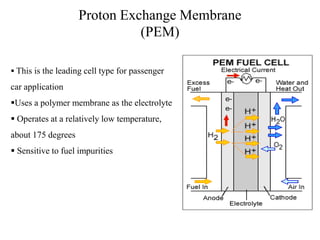
The document discusses hydrogen fuel cells, including their history, working principles, types, and applications. It provides the following key points:
- Hydrogen fuel cells were discovered in 1838 and work by combining hydrogen and oxygen to efficiently produce electricity and water. This is done through an electrochemical process without combustion.
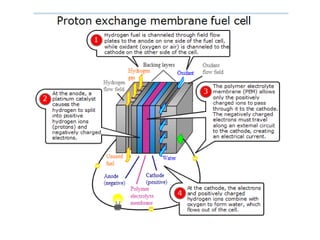
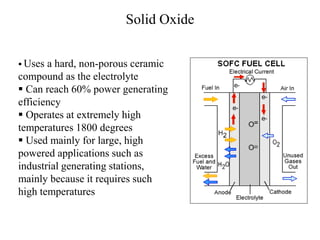
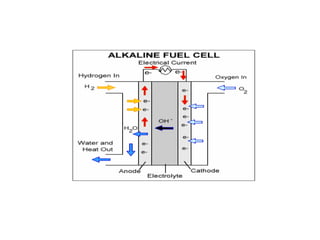
- There are several types of fuel cells including proton exchange membrane, phosphoric acid, solid oxide, and alkaline fuel cells, which differ in their electrolyte and operating temperatures.
- Fuel cells have many potential applications from transportation to backup power and are more efficient than combustion engines. They produce only water and heat as byproducts, making them a cleaner alternative to fossil fuels.