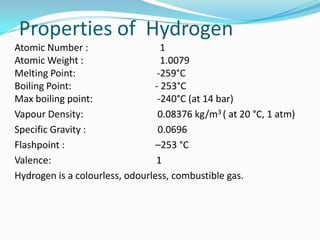
Hydrogen is the most abundant element in the universe but does not exist naturally on Earth. It has potential as a clean fuel for vehicles and devices. Currently it is mainly produced from methane, but can also be generated through electrolysis and other methods. Hydrogen is colorless, odorless and highly flammable. It can power vehicles and devices through combustion or in fuel cells, which generate electricity through electrochemical reactions with oxygen and have higher efficiency than combustion engines. Widespread use of hydrogen faces challenges including lack of infrastructure and need for cost reductions in production and fuel cells.