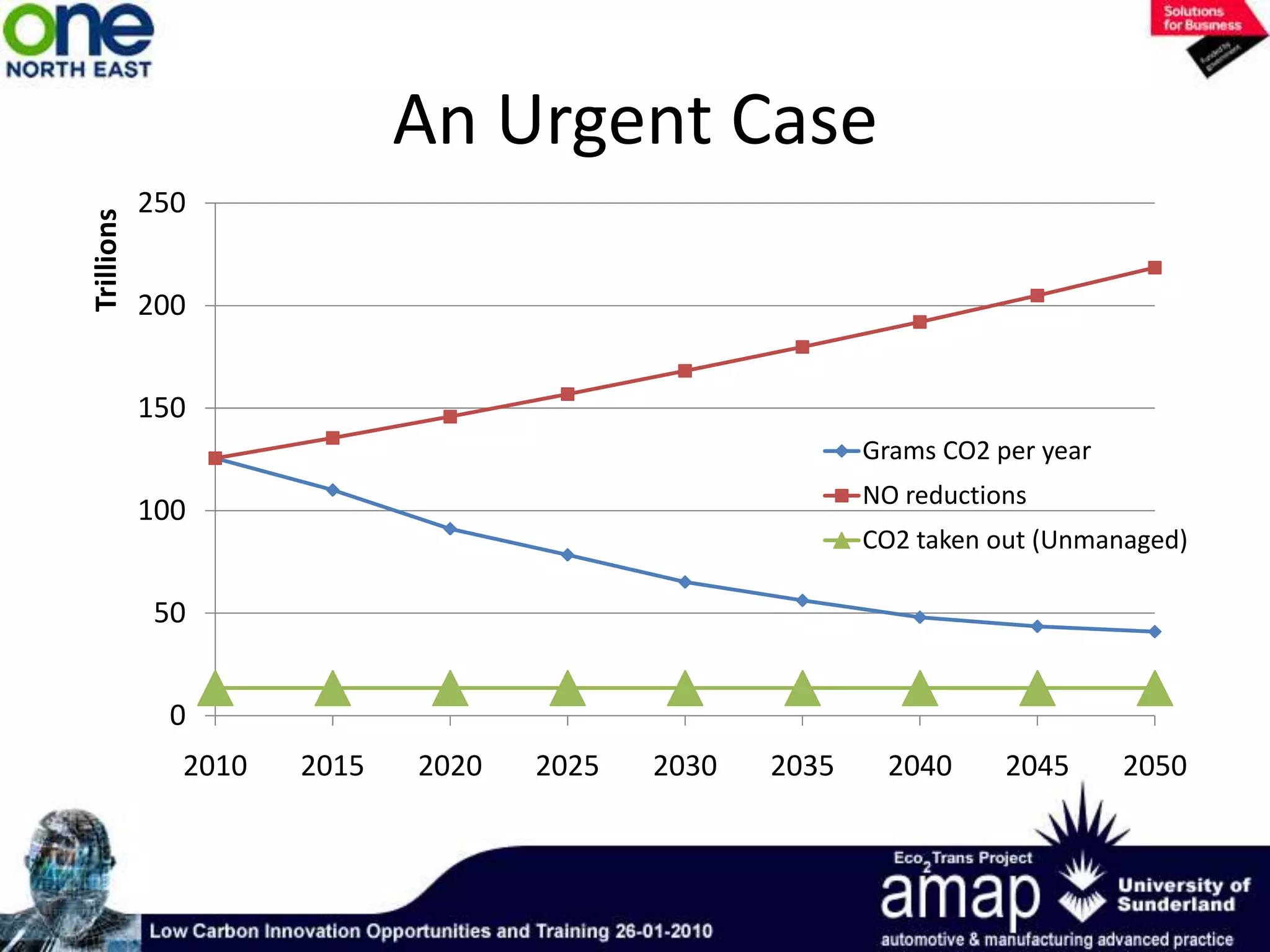
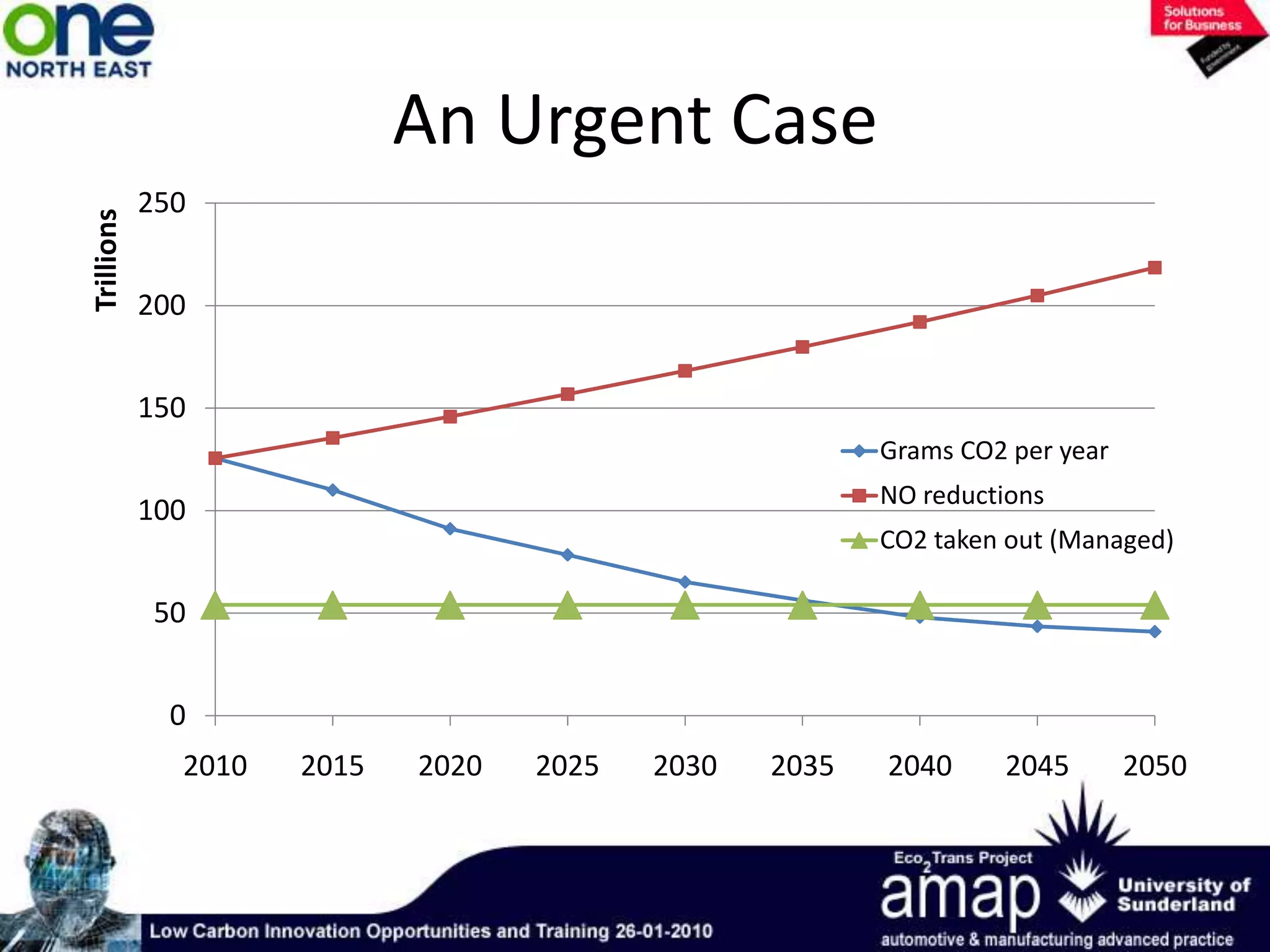
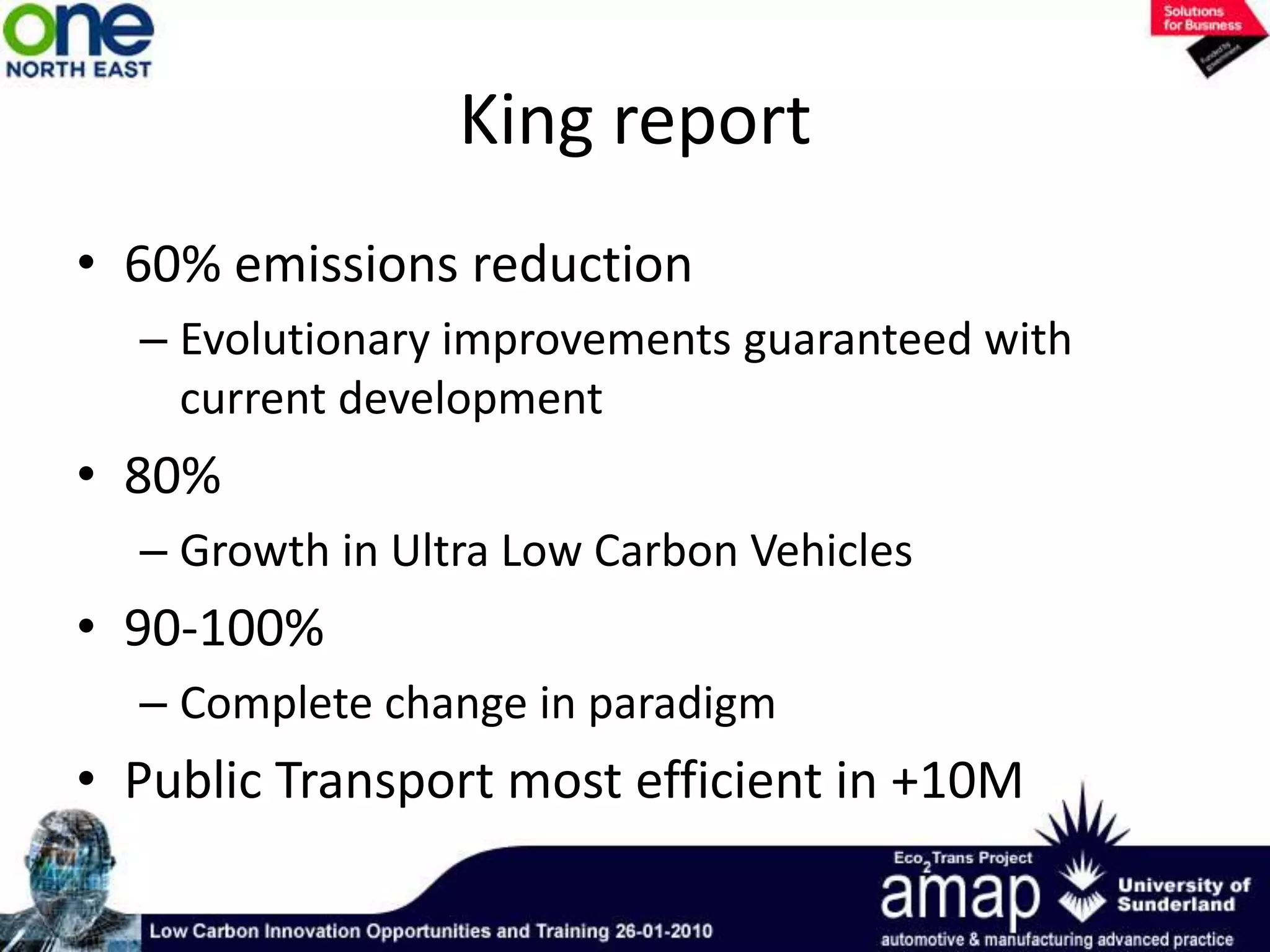
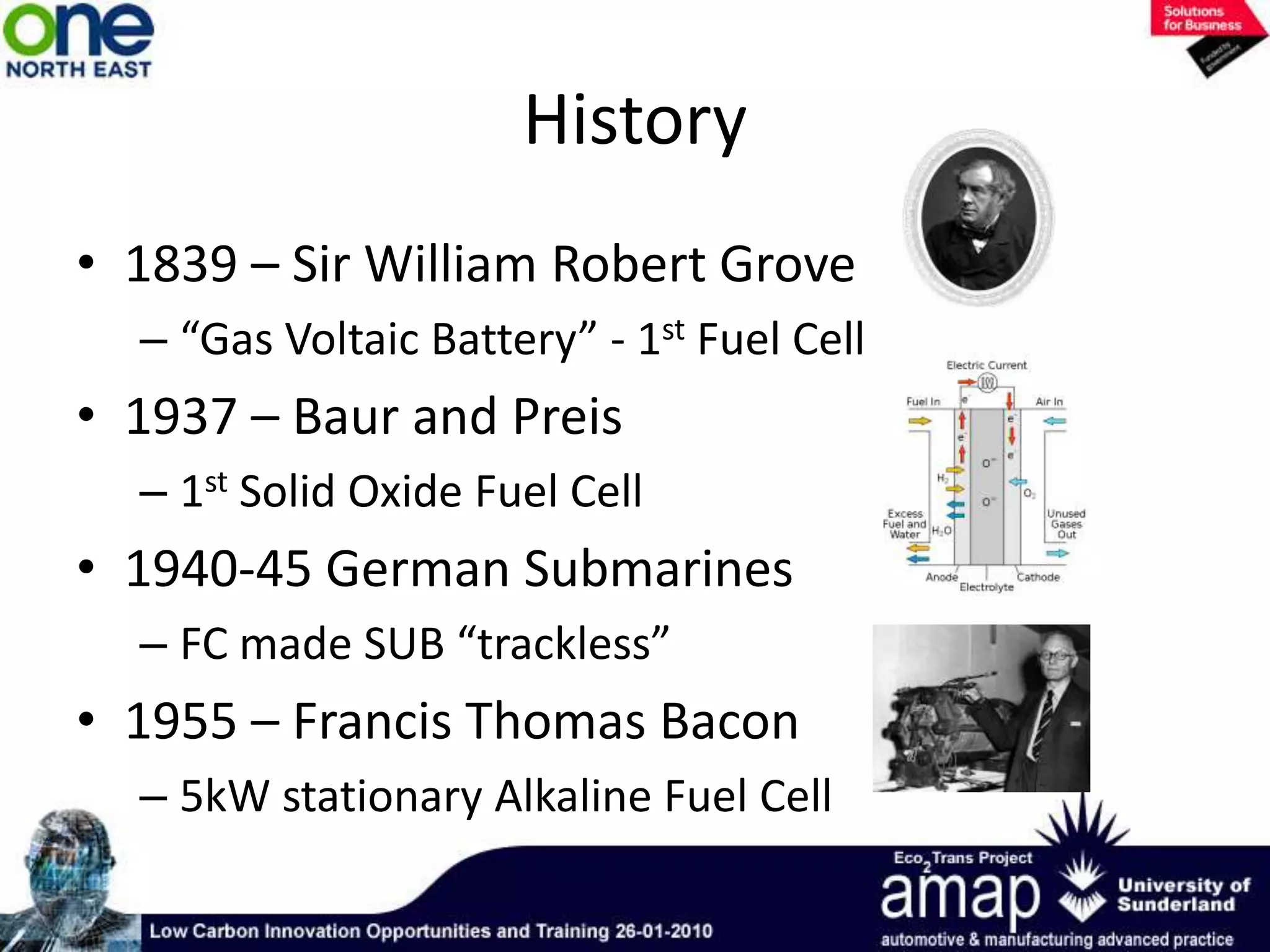
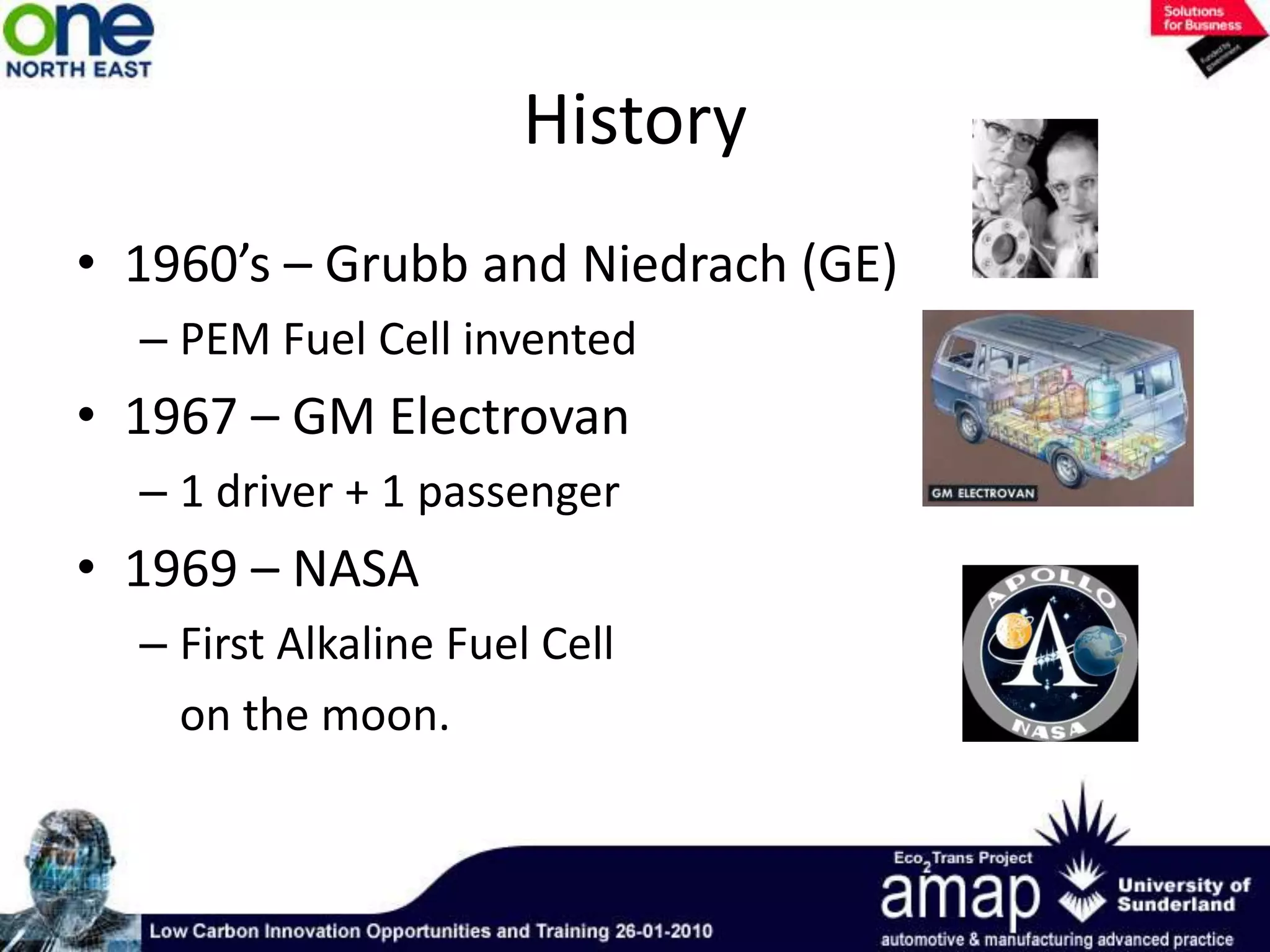
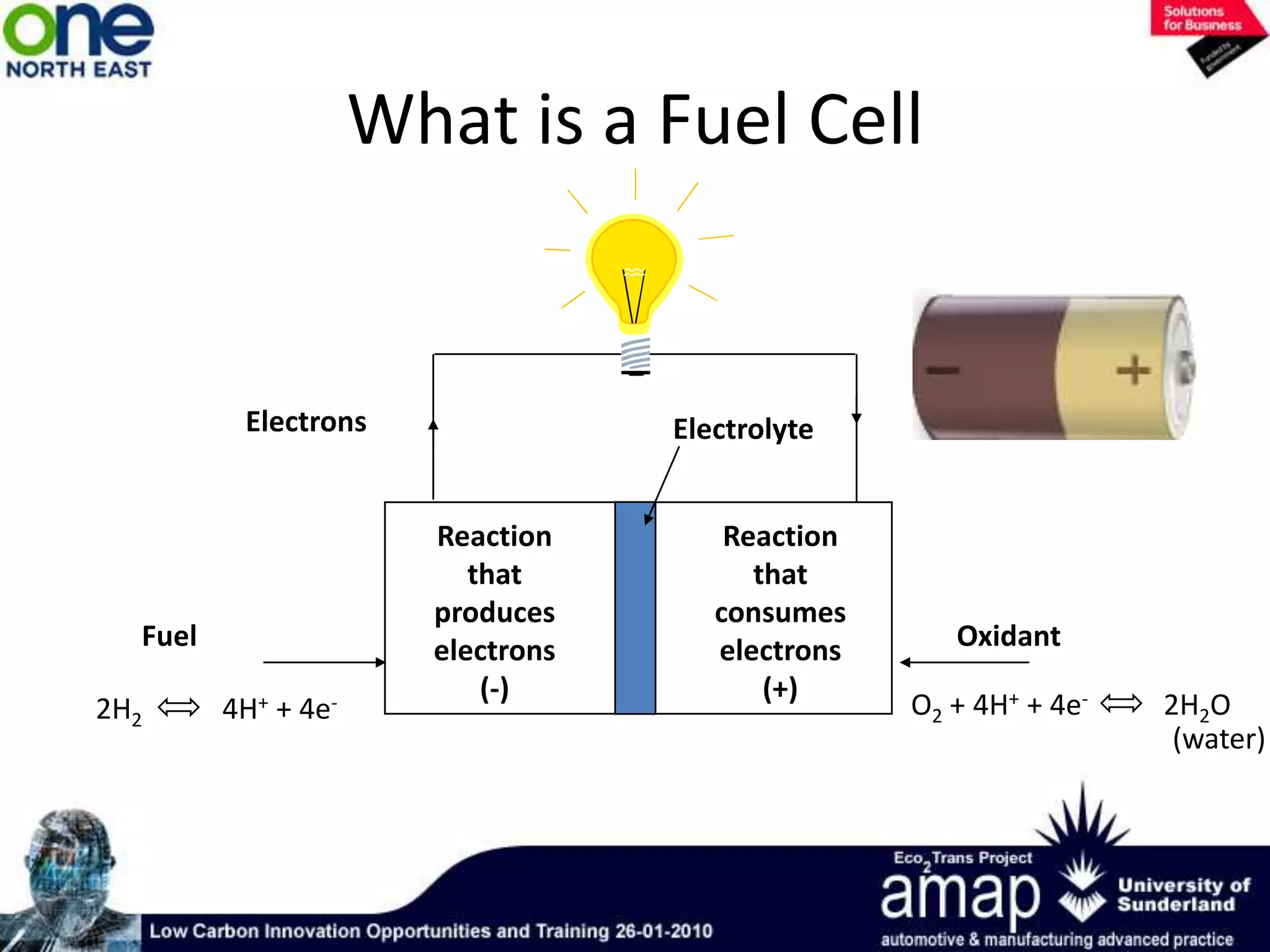
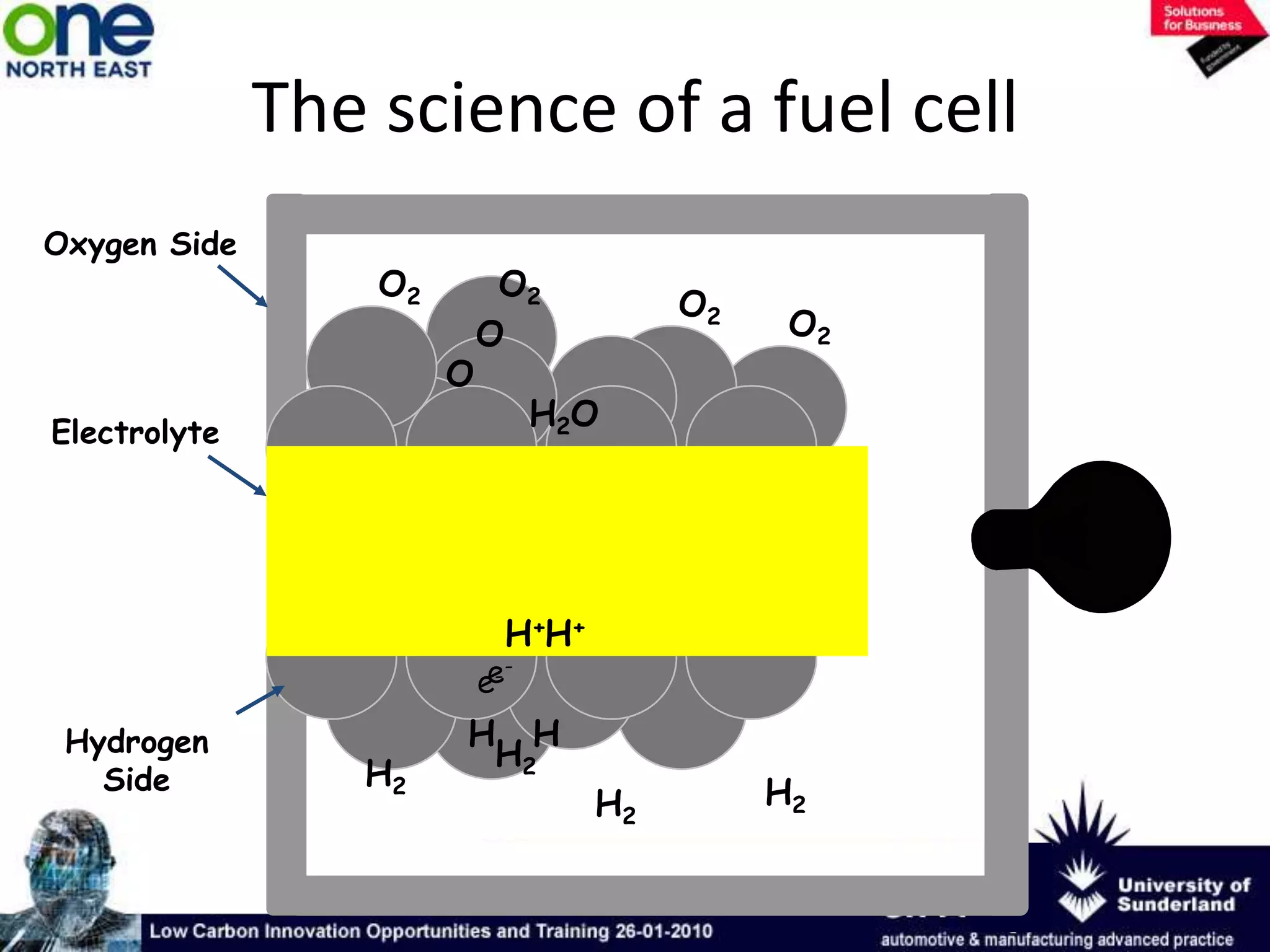
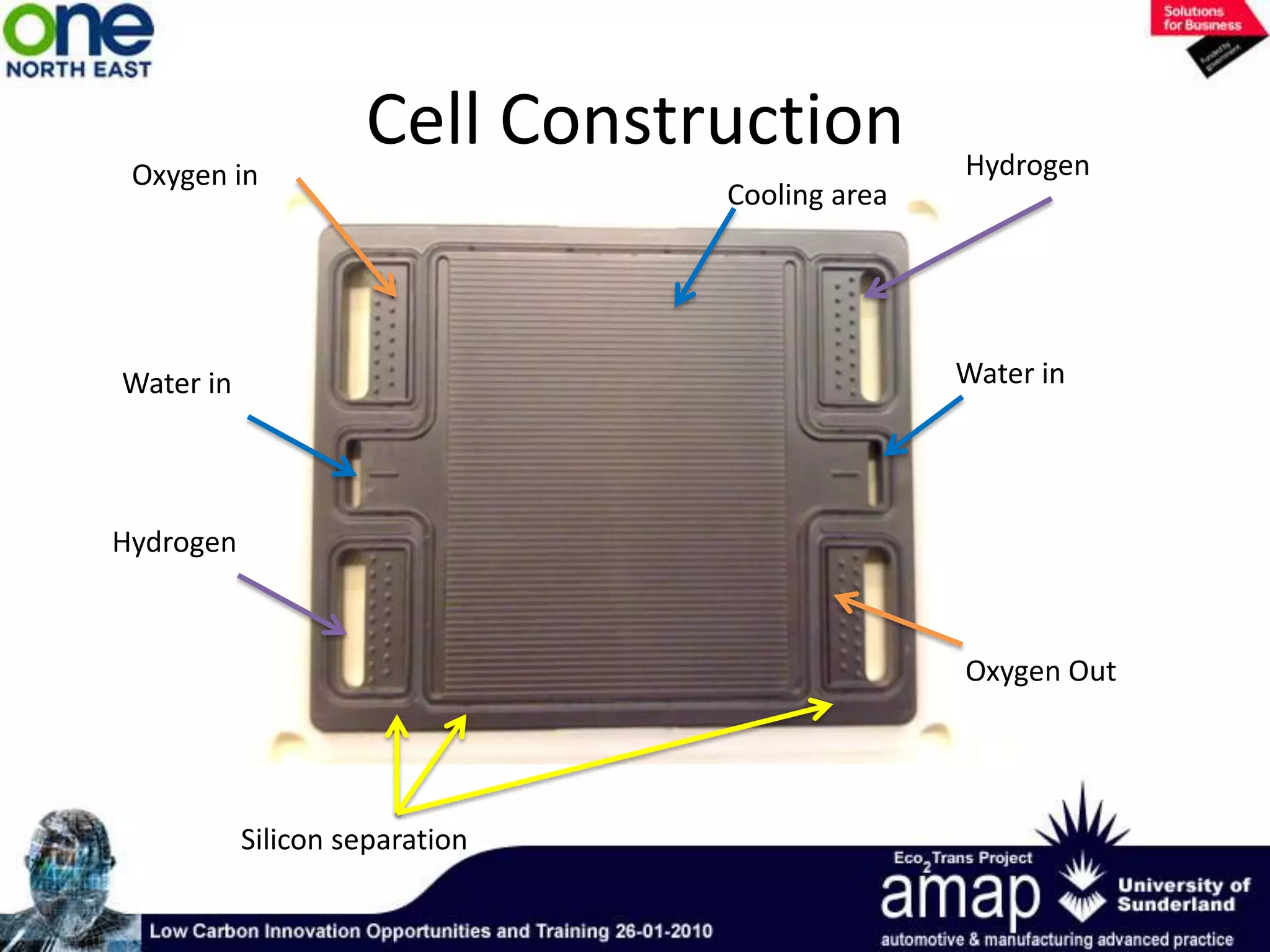
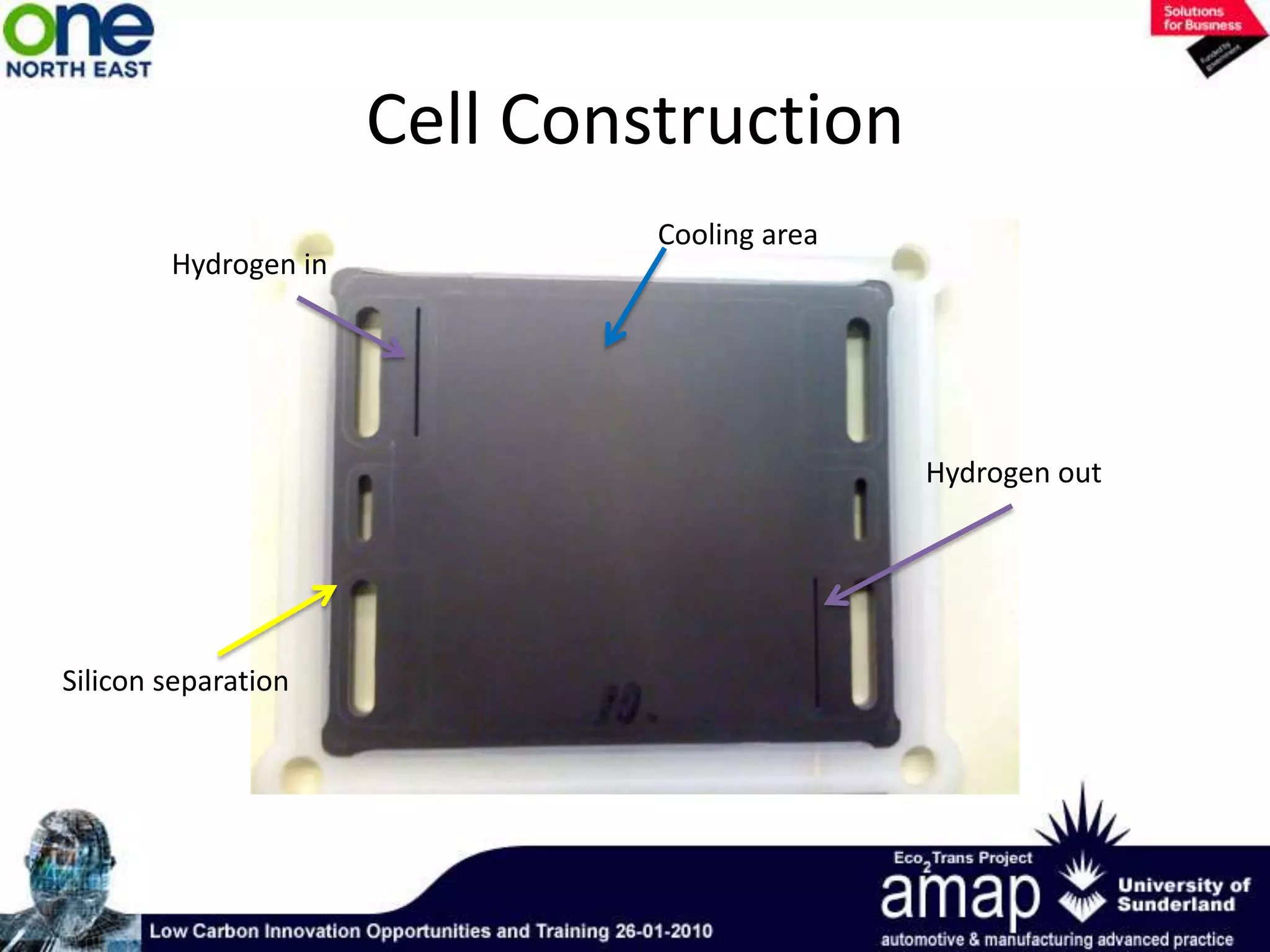
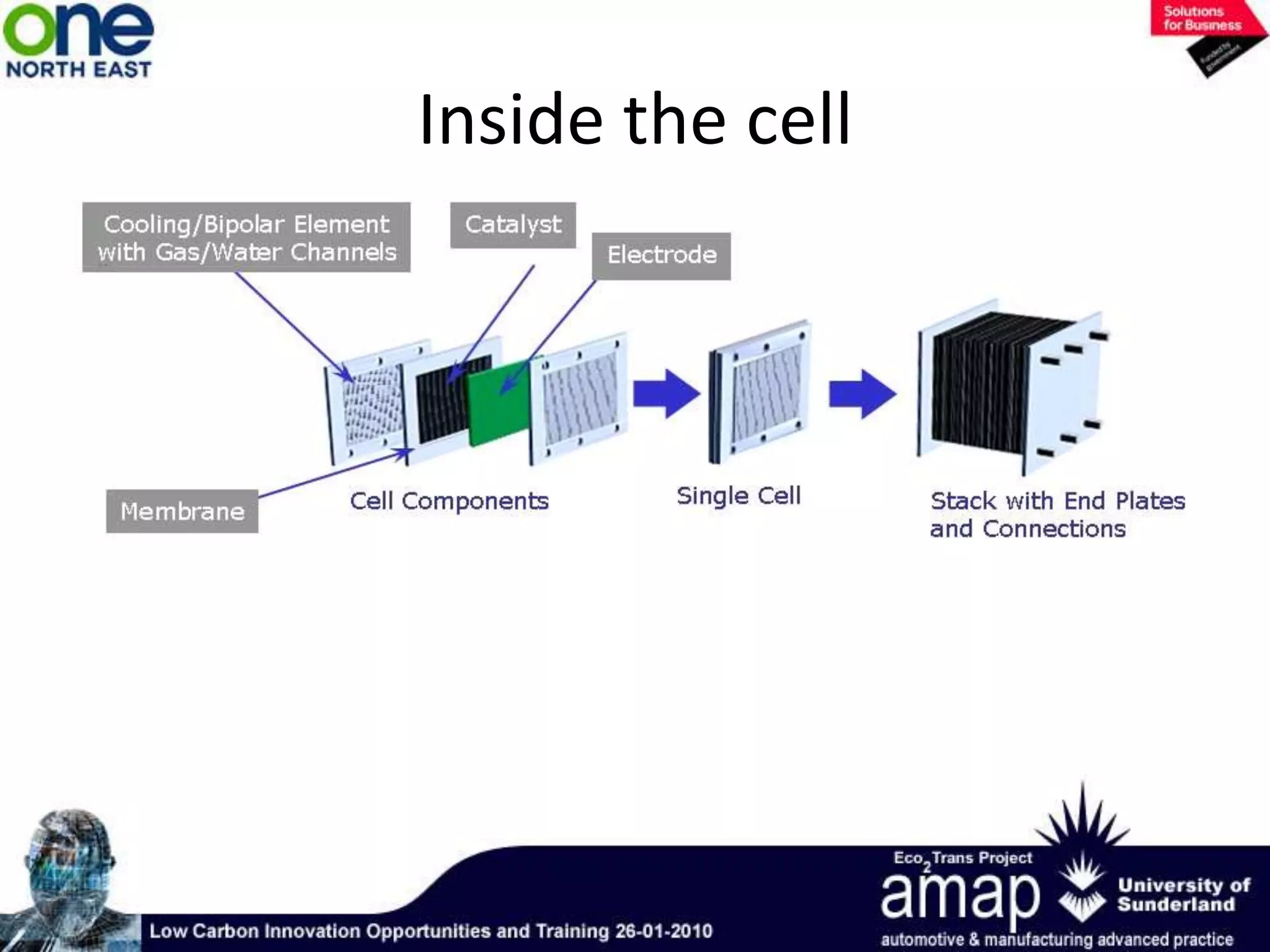
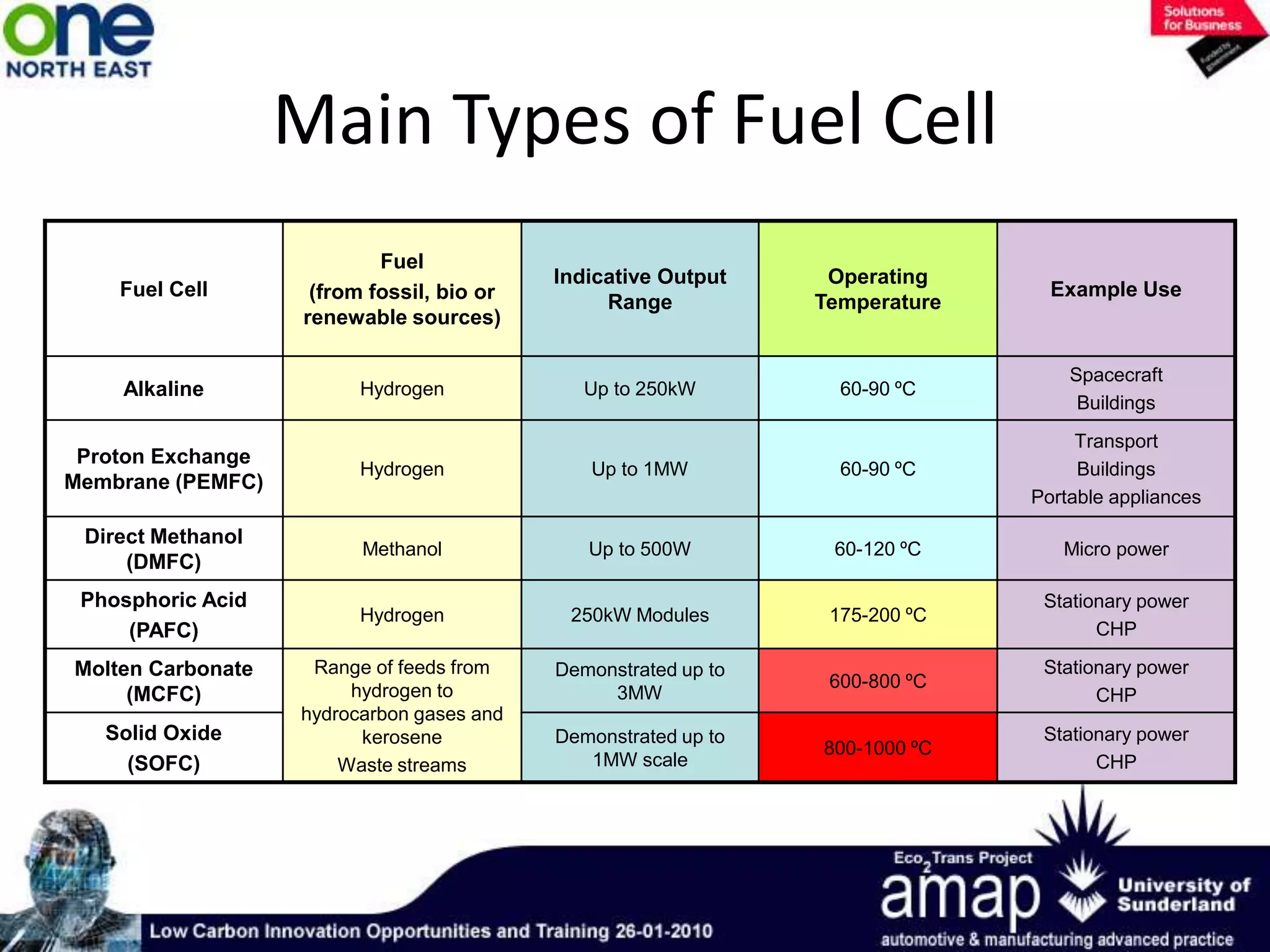
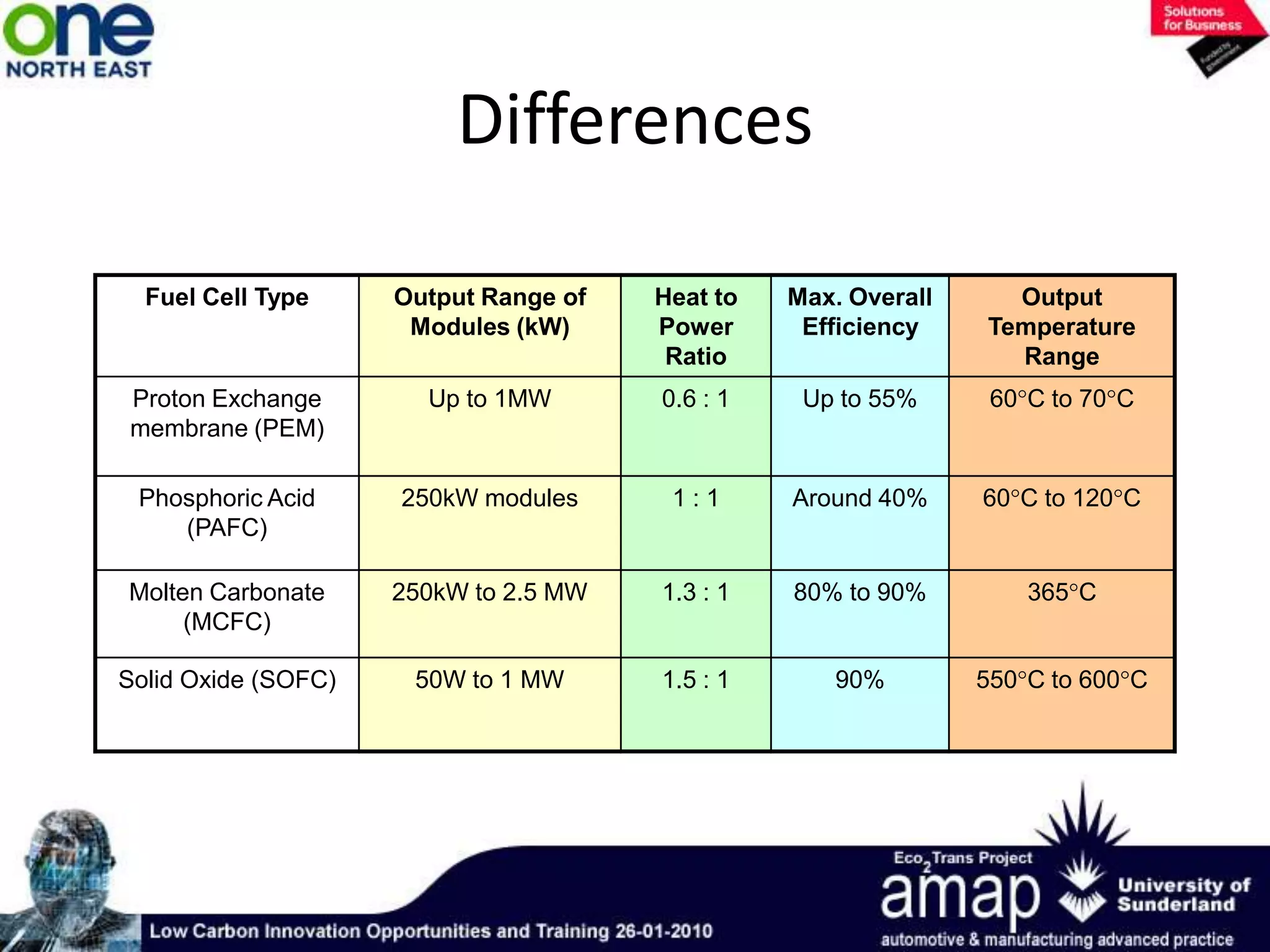
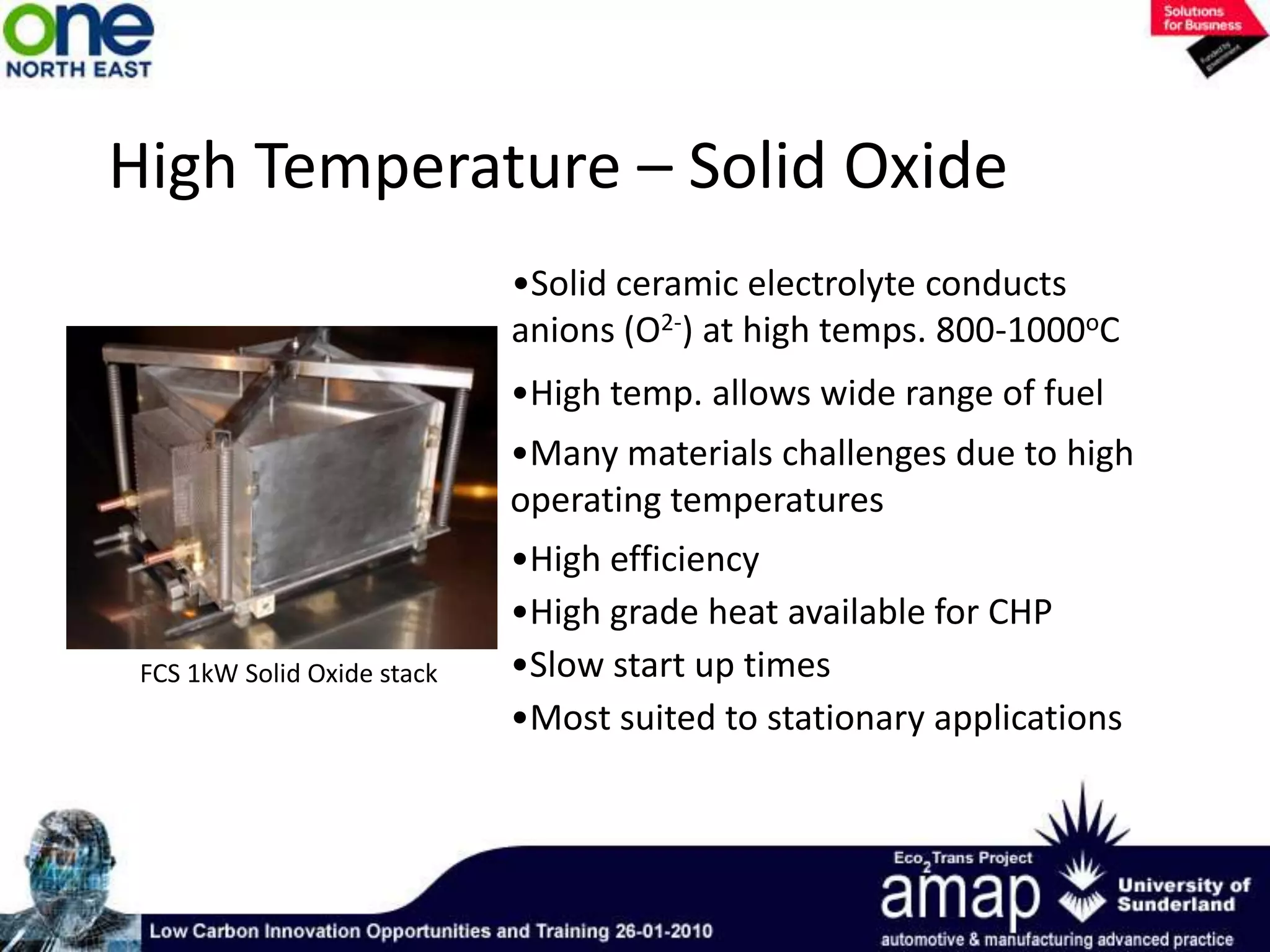
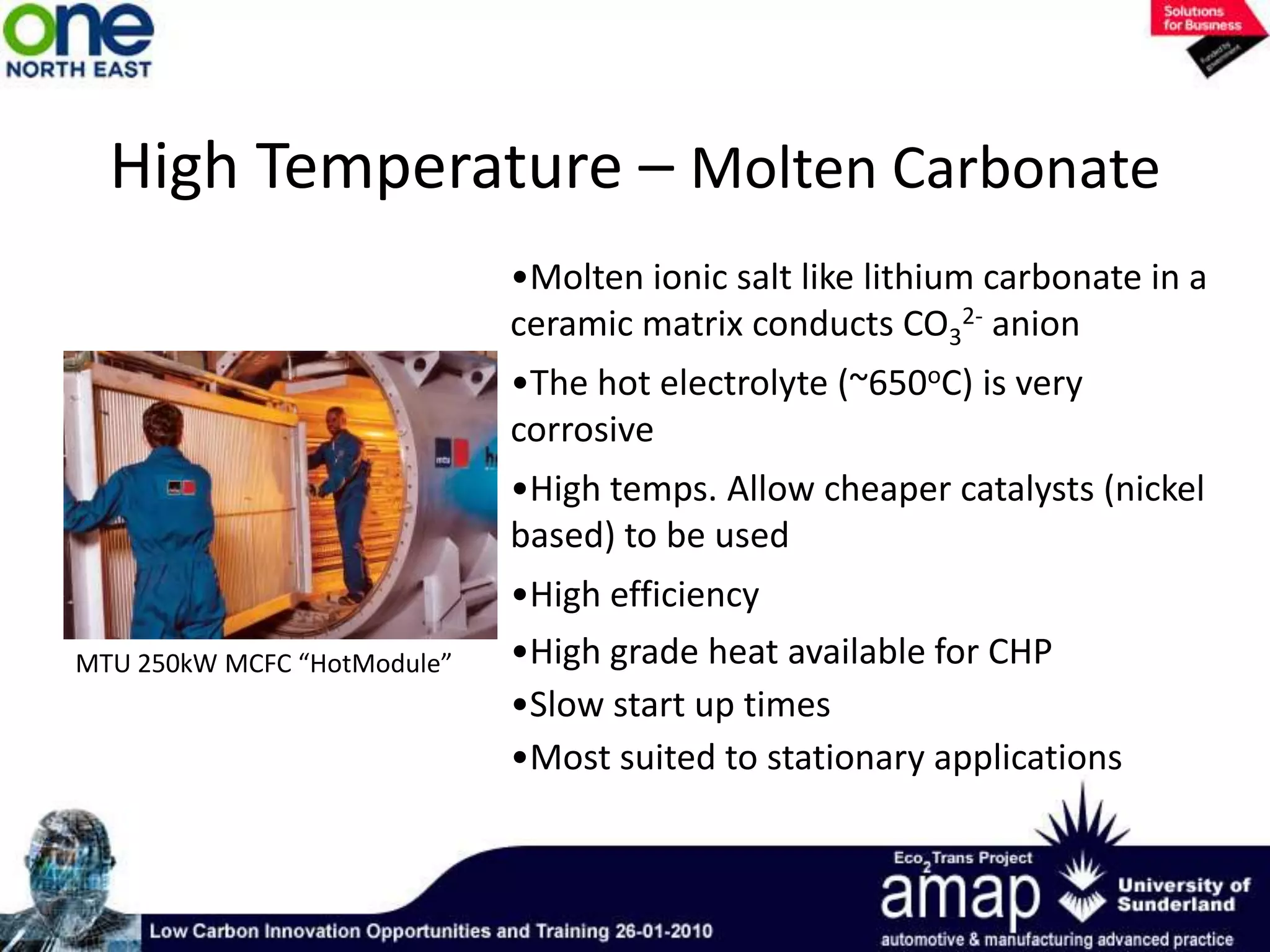
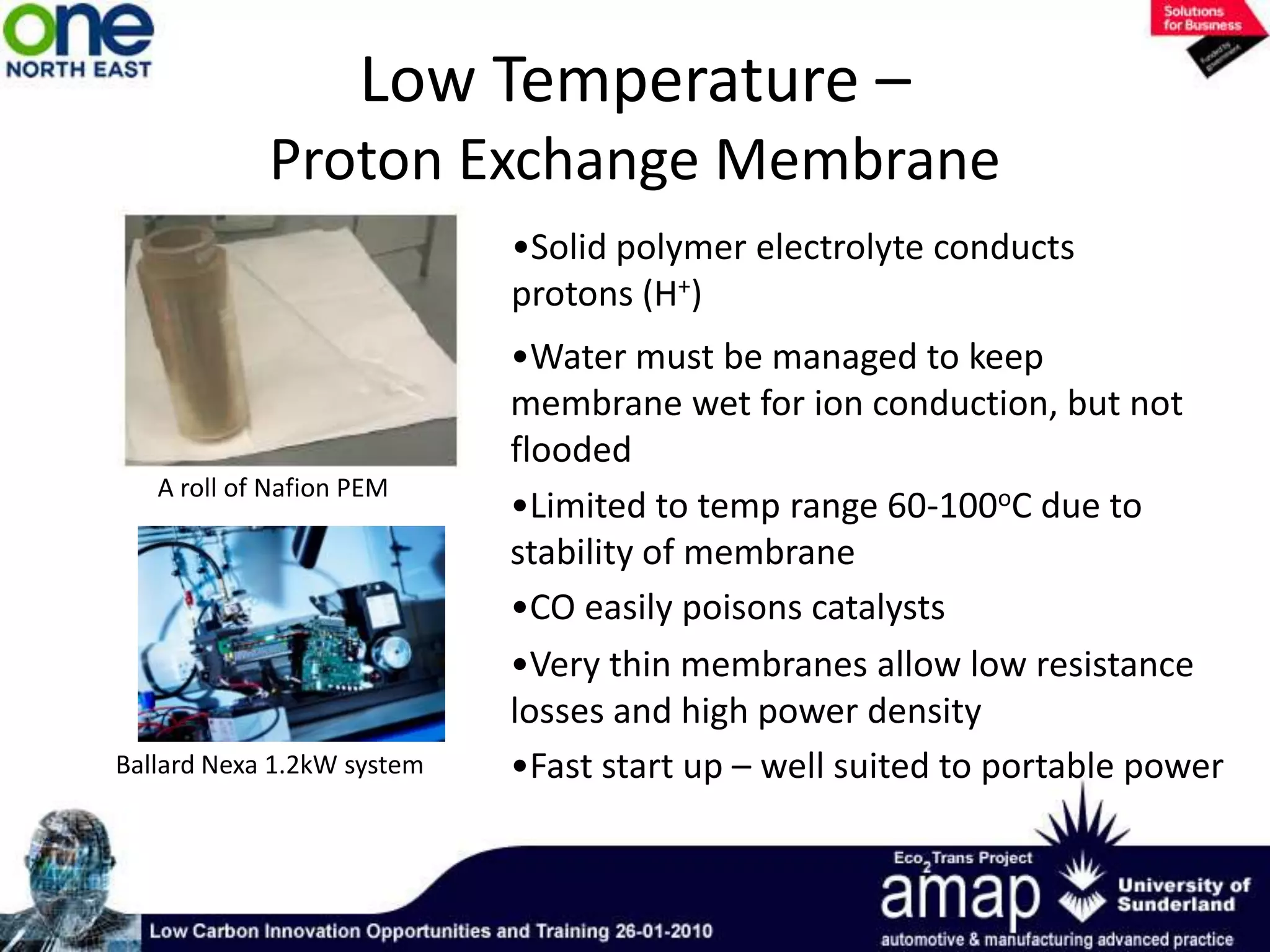
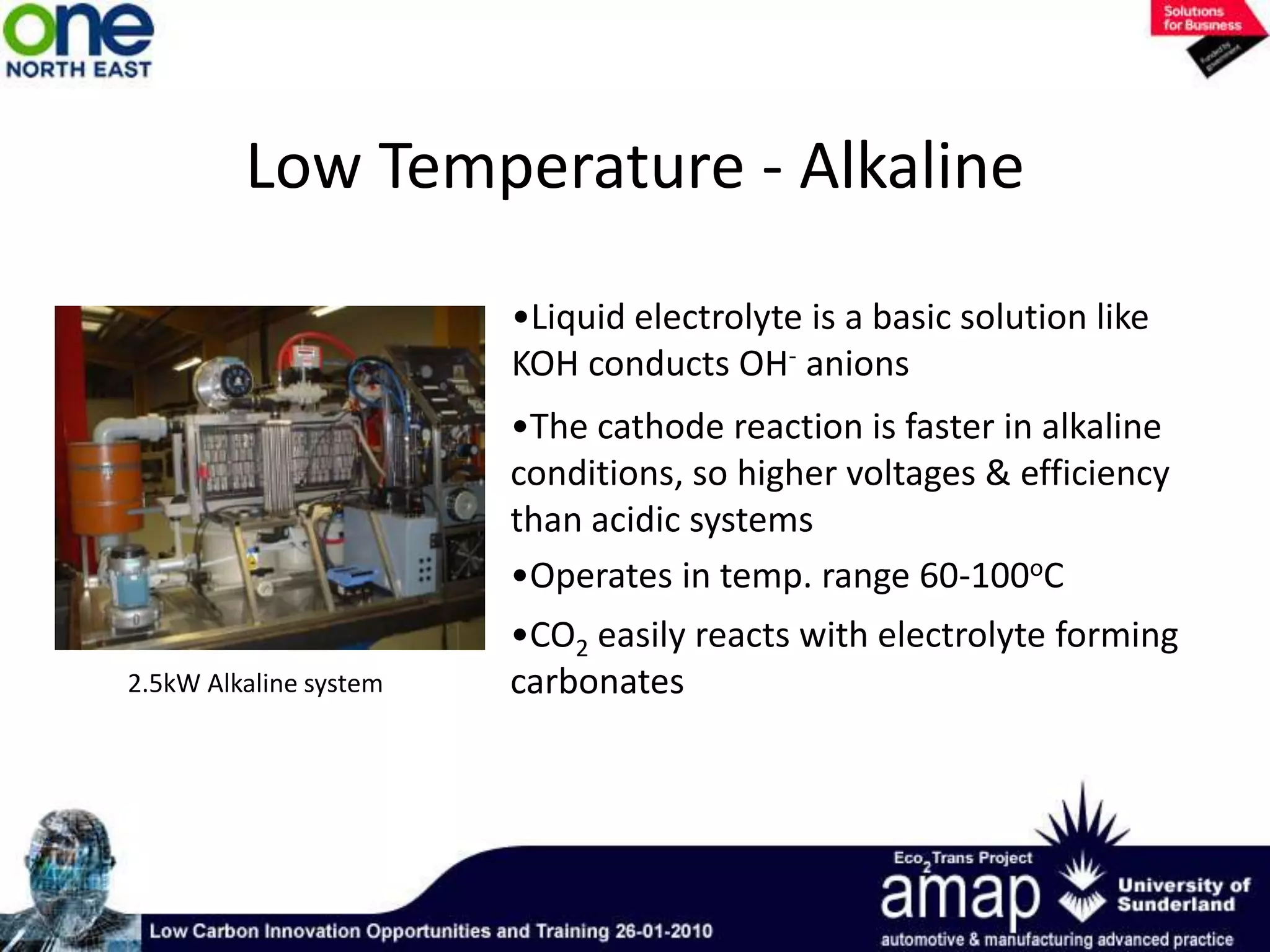
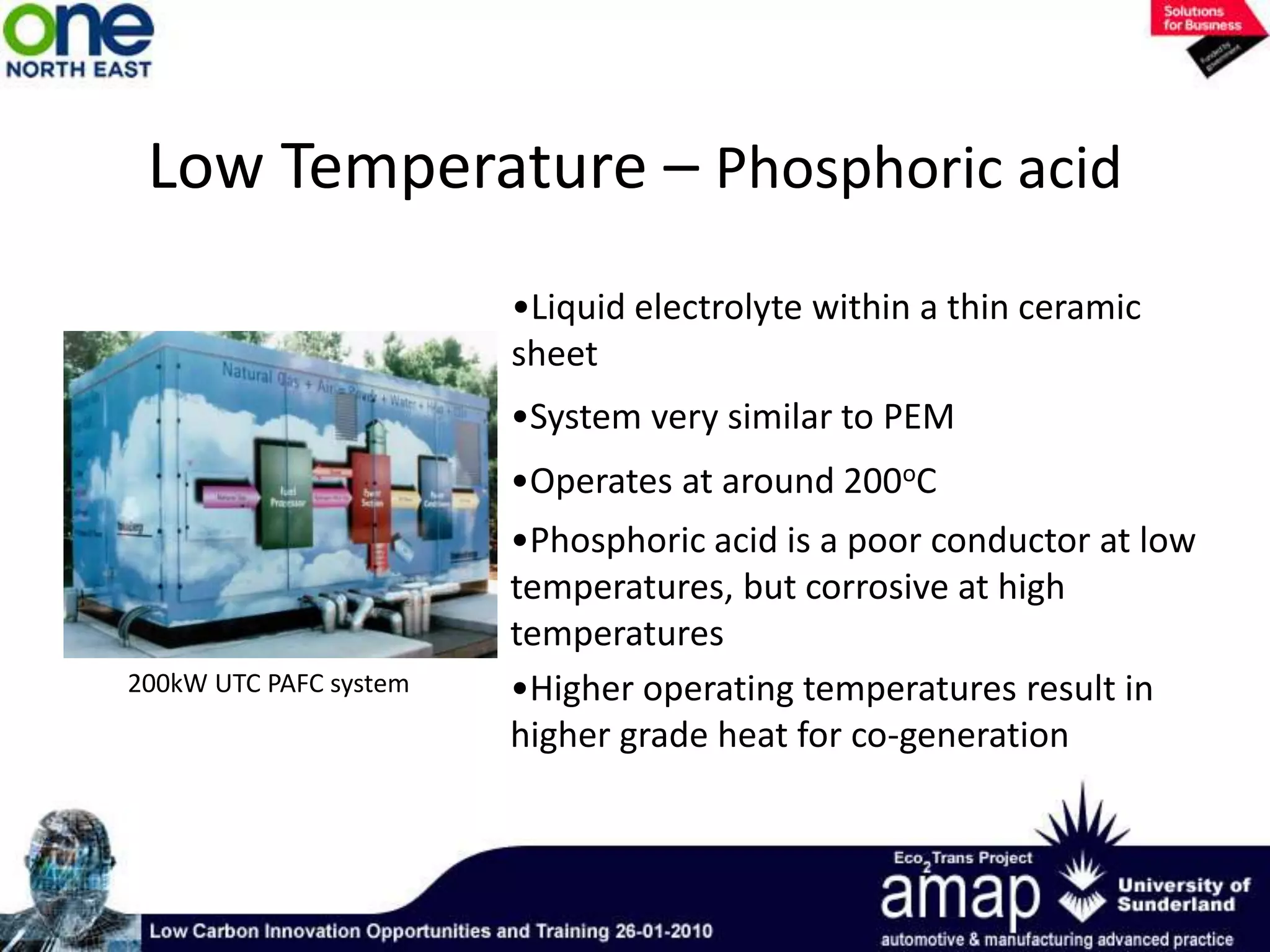
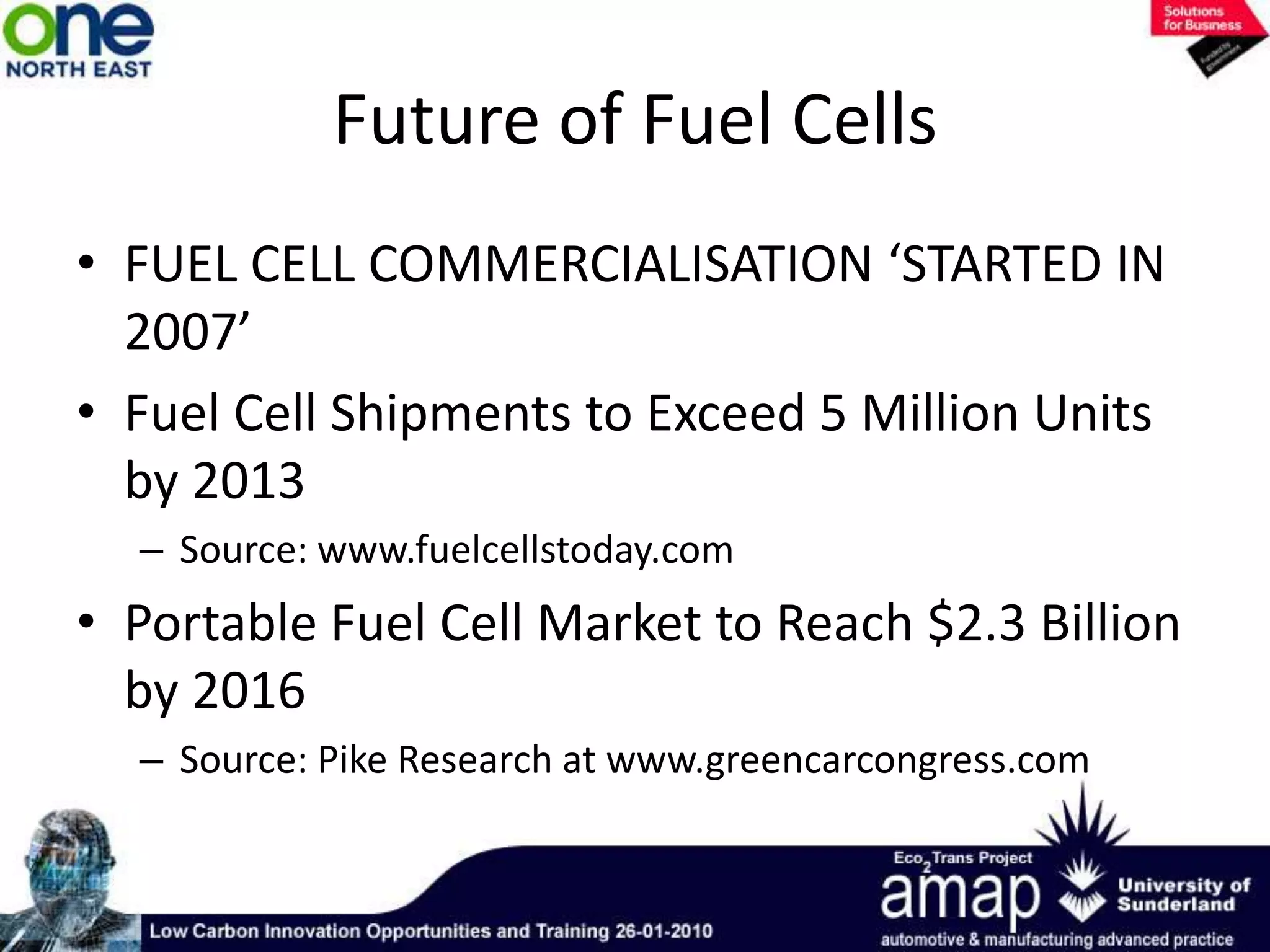
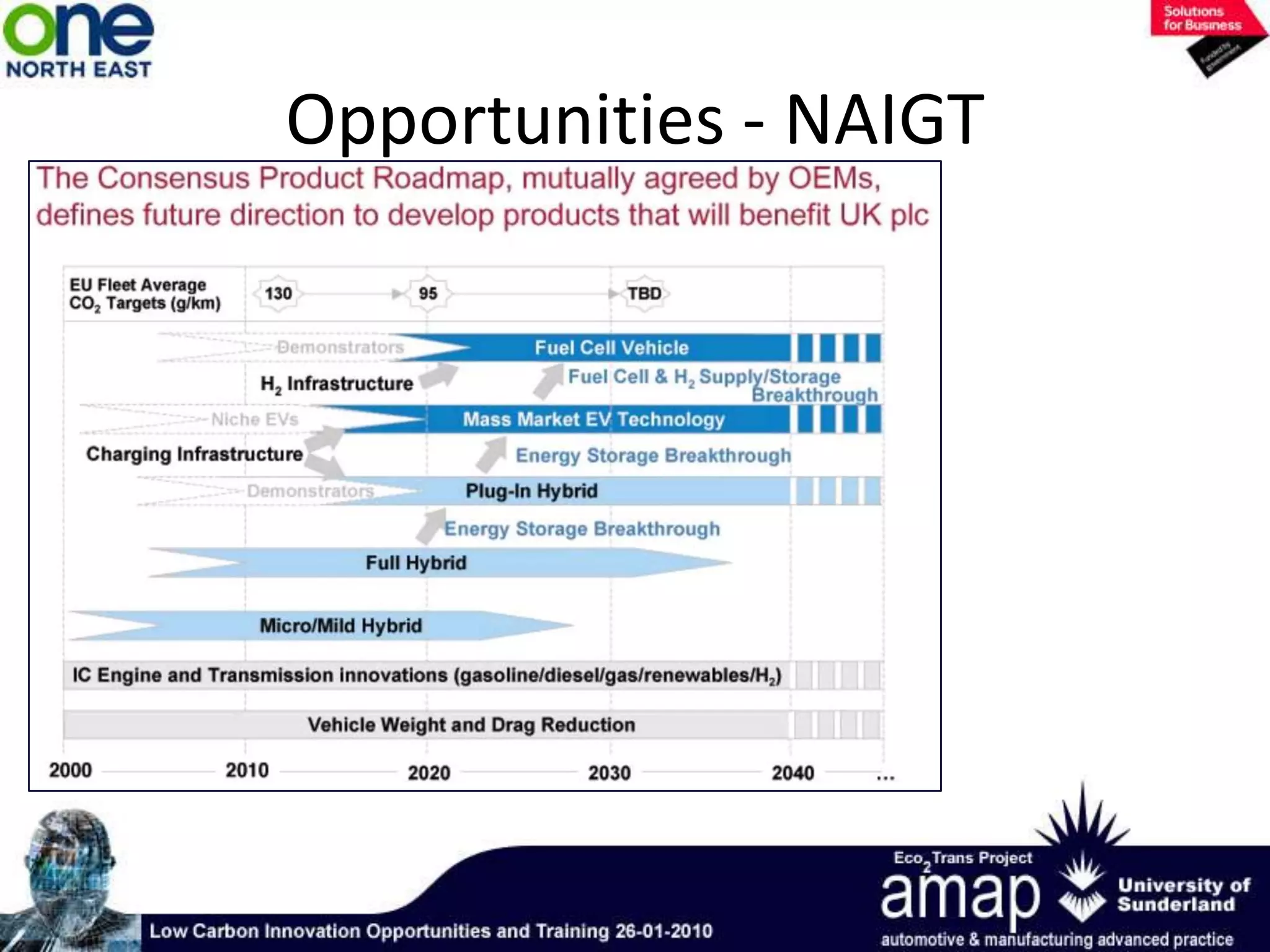
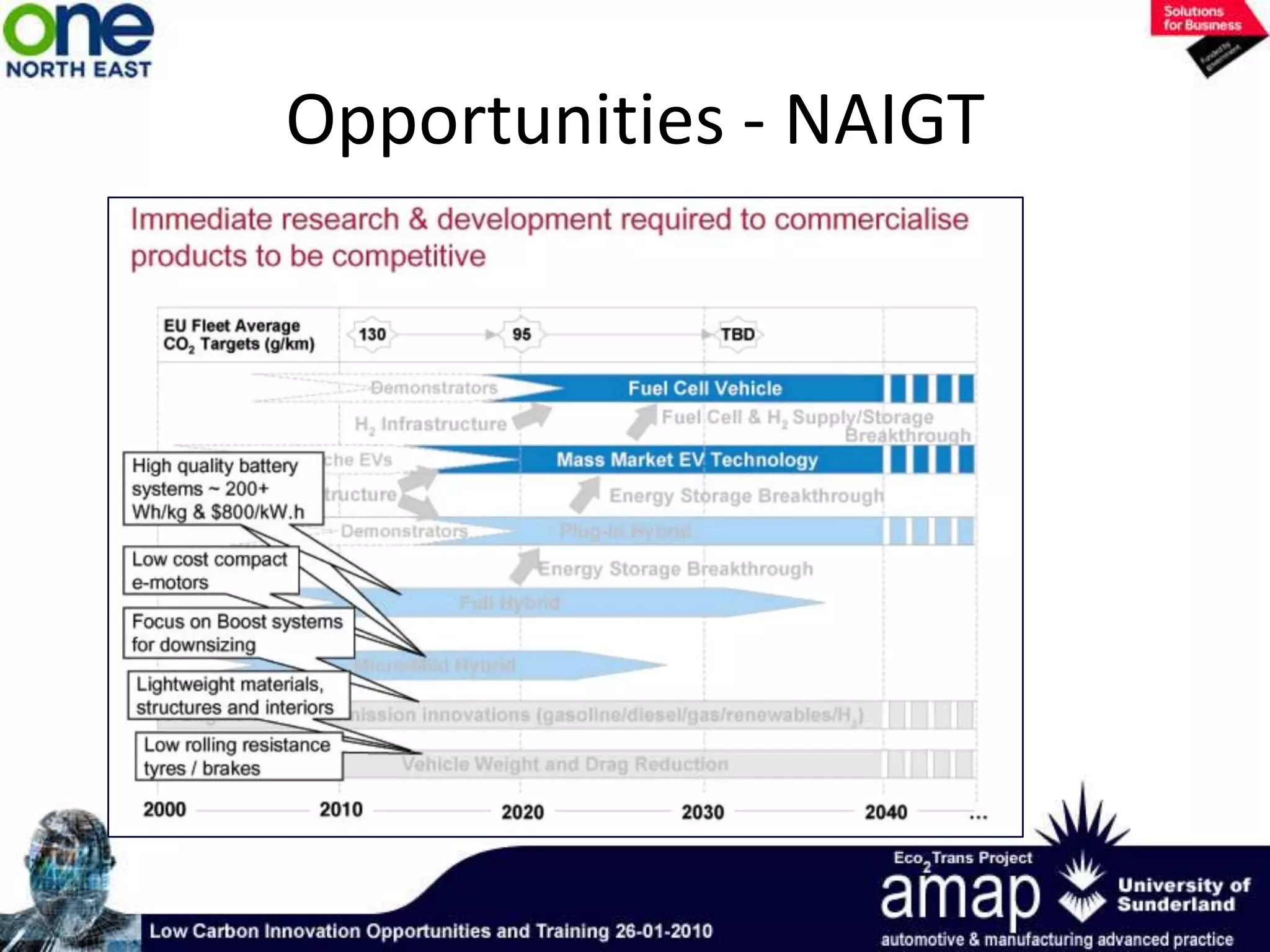
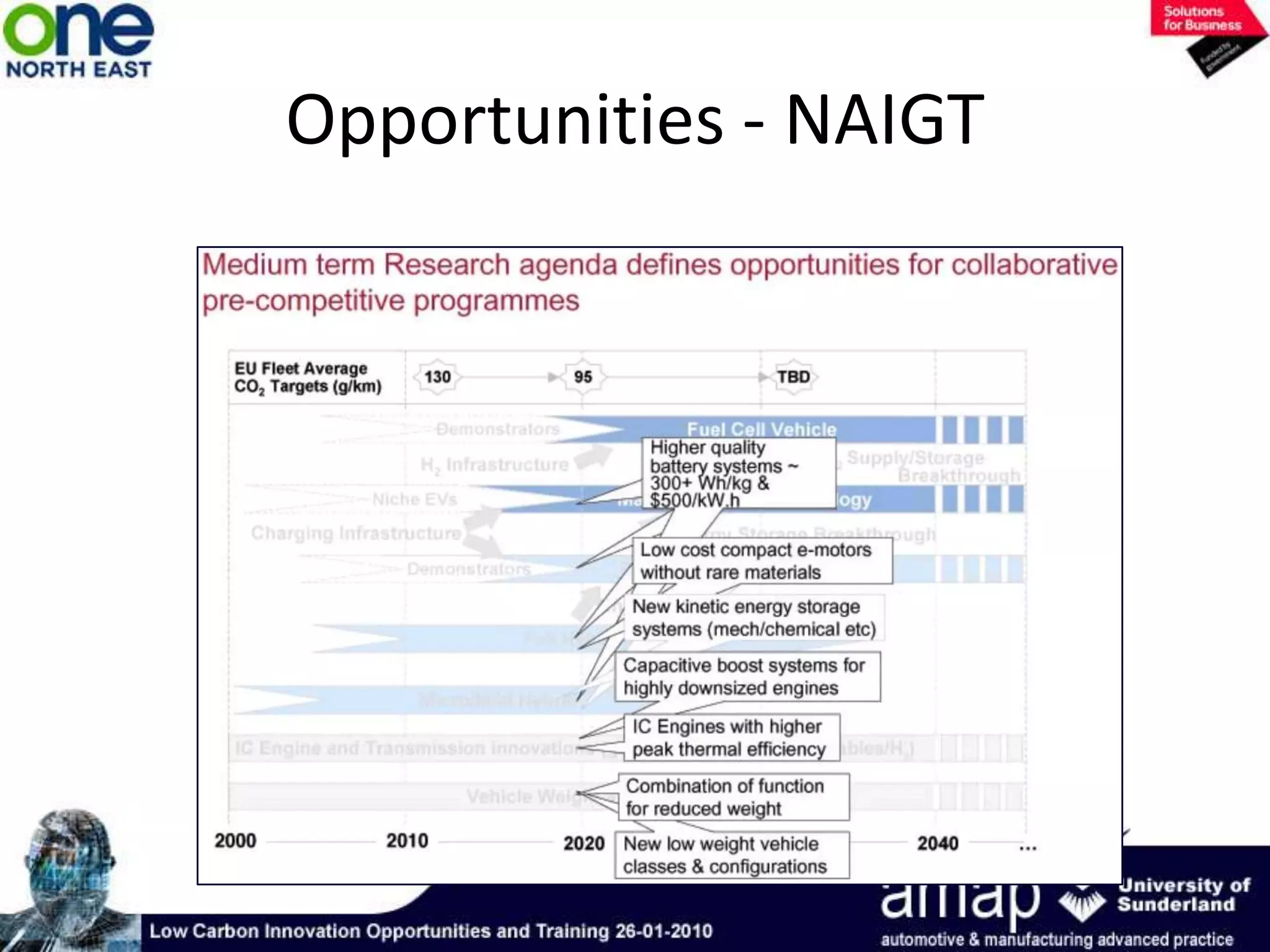
This document summarizes the history and future of hydrogen as a fuel source and fuel cells. It discusses how fuel cells work by converting the chemical energy in hydrogen into electricity through an electrochemical reaction. Different types of fuel cells are described, including proton exchange membrane, alkaline, phosphoric acid, molten carbonate, and solid oxide fuel cells. Applications for fuel cells include transportation, portable power devices, and stationary power generation. The document concludes that the commercialization of fuel cells is increasing, with projections of millions of fuel cell shipments by the next decade, and opportunities for further innovation in areas like hydrogen generation and storage.