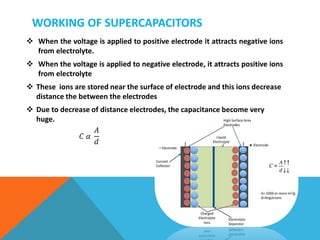
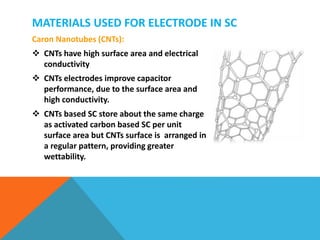
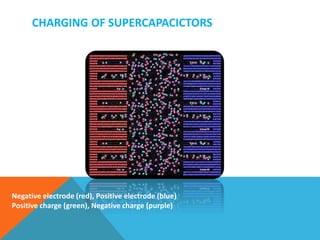
The document discusses nanomaterials used for electrodes in supercapacitors. It begins by explaining the basic construction and working of supercapacitors, which store charge electrostatically at the electrode-electrolyte interface. Common nanomaterial electrodes mentioned include activated carbon, carbon aerogel, graphene, and carbon nanotubes due to their high surface areas and conductivities. These properties allow for high capacitance and energy density in supercapacitors.