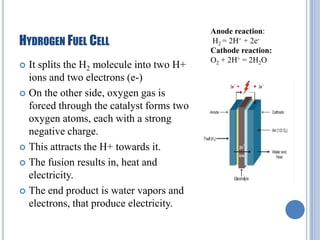
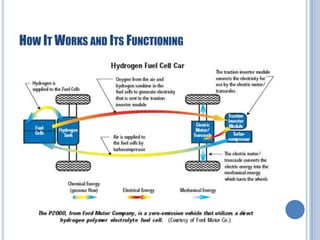
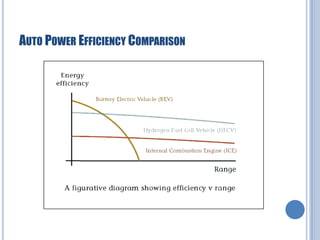
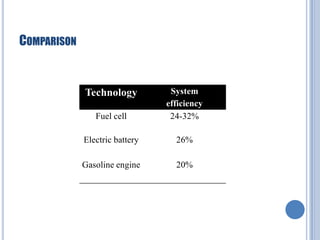
This document discusses hydrogen fuel cells for use in automobiles. It begins with an introduction to fuel cells, explaining that they generate electricity through an electrochemical reaction between hydrogen and oxygen without combustion. The parts of a typical fuel cell are then described, including the anode, cathode, electrolyte, and catalyst. The document goes on to explain how a hydrogen fuel cell works to split hydrogen and oxygen and generate electricity, water, and heat. It notes that hydrogen fuel cells could power electric vehicles without pollution. The document concludes by discussing challenges like hydrogen storage and costs, but envisions potential benefits if the technology is improved.