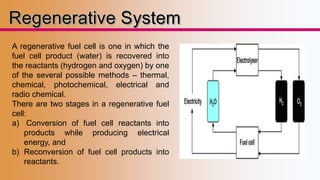
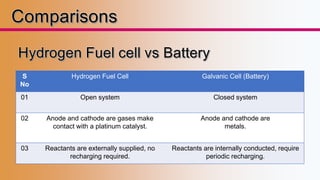
This presentation provides an overview of fuel cells, including their design, operation, types, advantages/disadvantages, and applications. It discusses how fuel cells work by electrochemically combining hydrogen and oxygen to produce electricity and water. The document outlines different classifications of fuel cells based on temperature, electrolyte, physical state of fuel used. It also compares fuel cells to batteries and internal combustion engines. Recent developments and applications of fuel cells in areas like transportation, power generation, and specialty uses are presented.