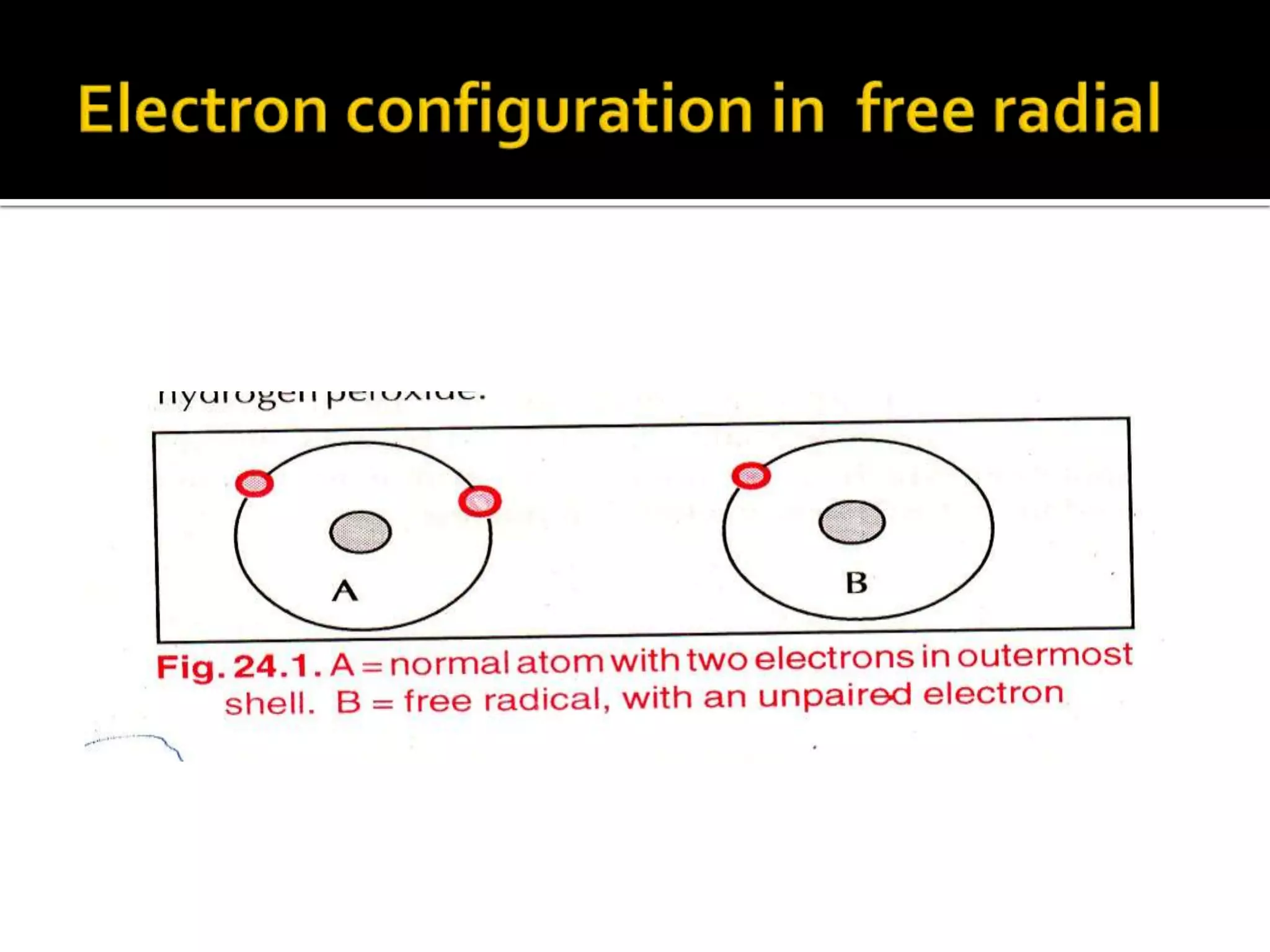
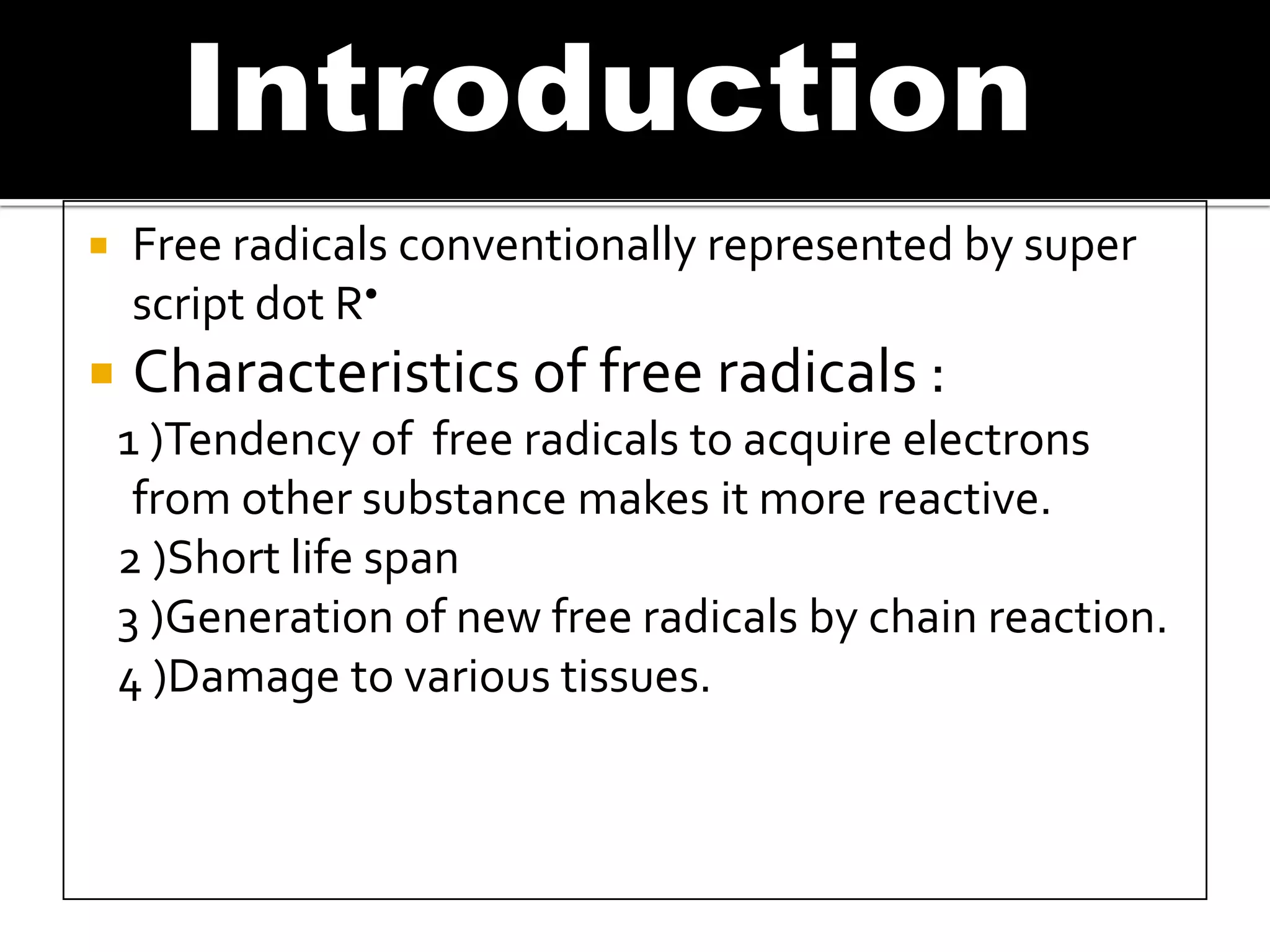
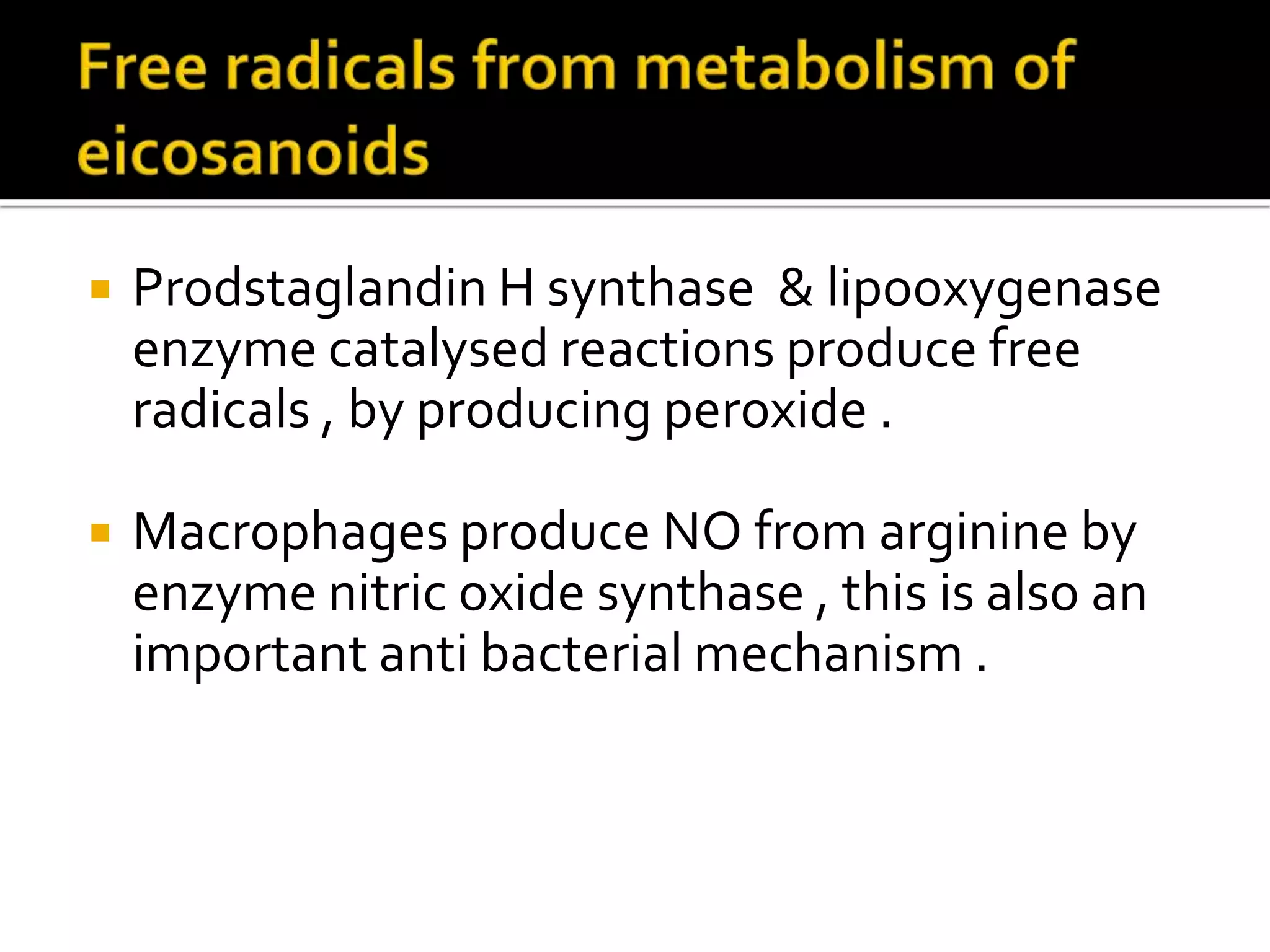
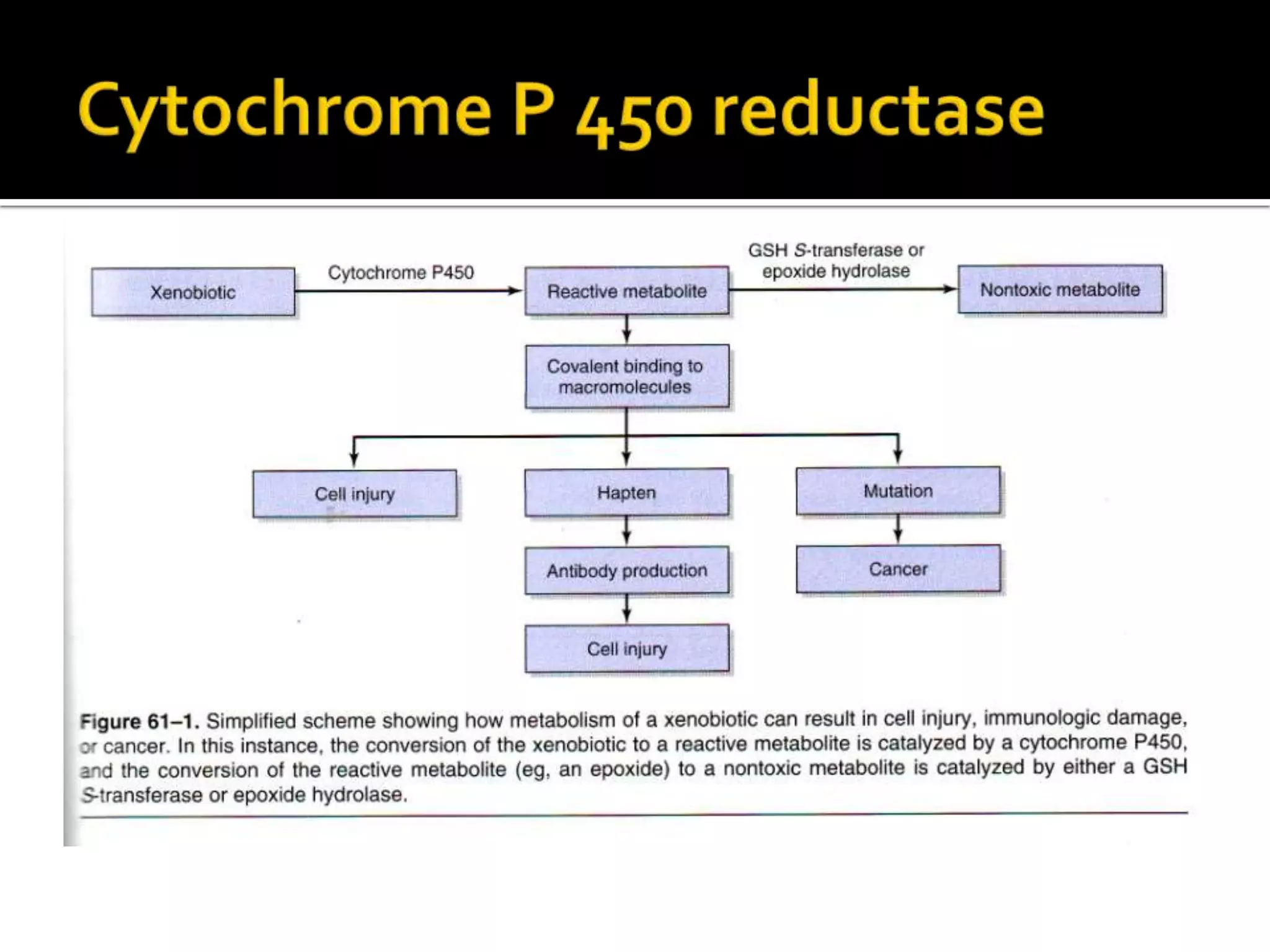
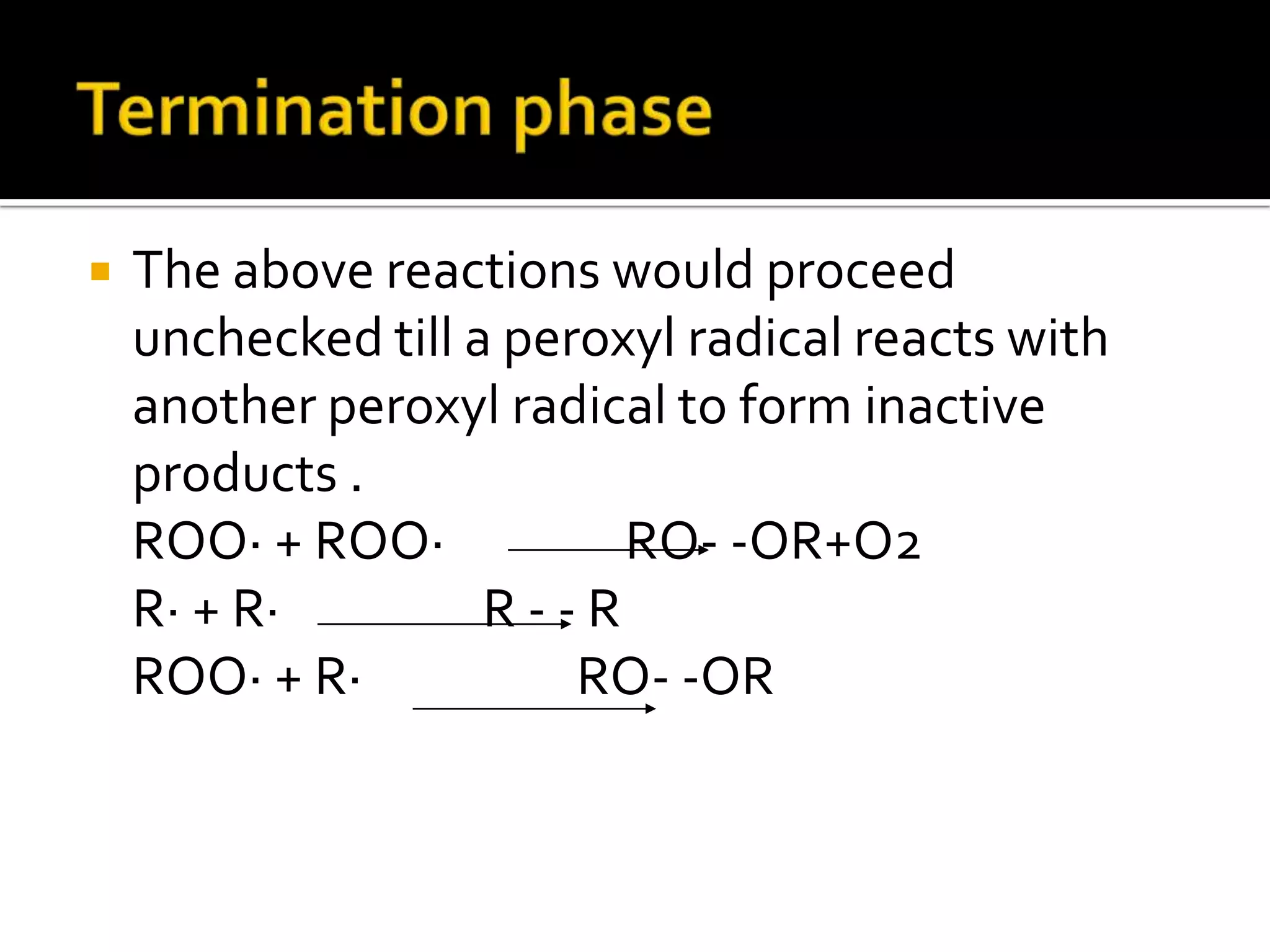
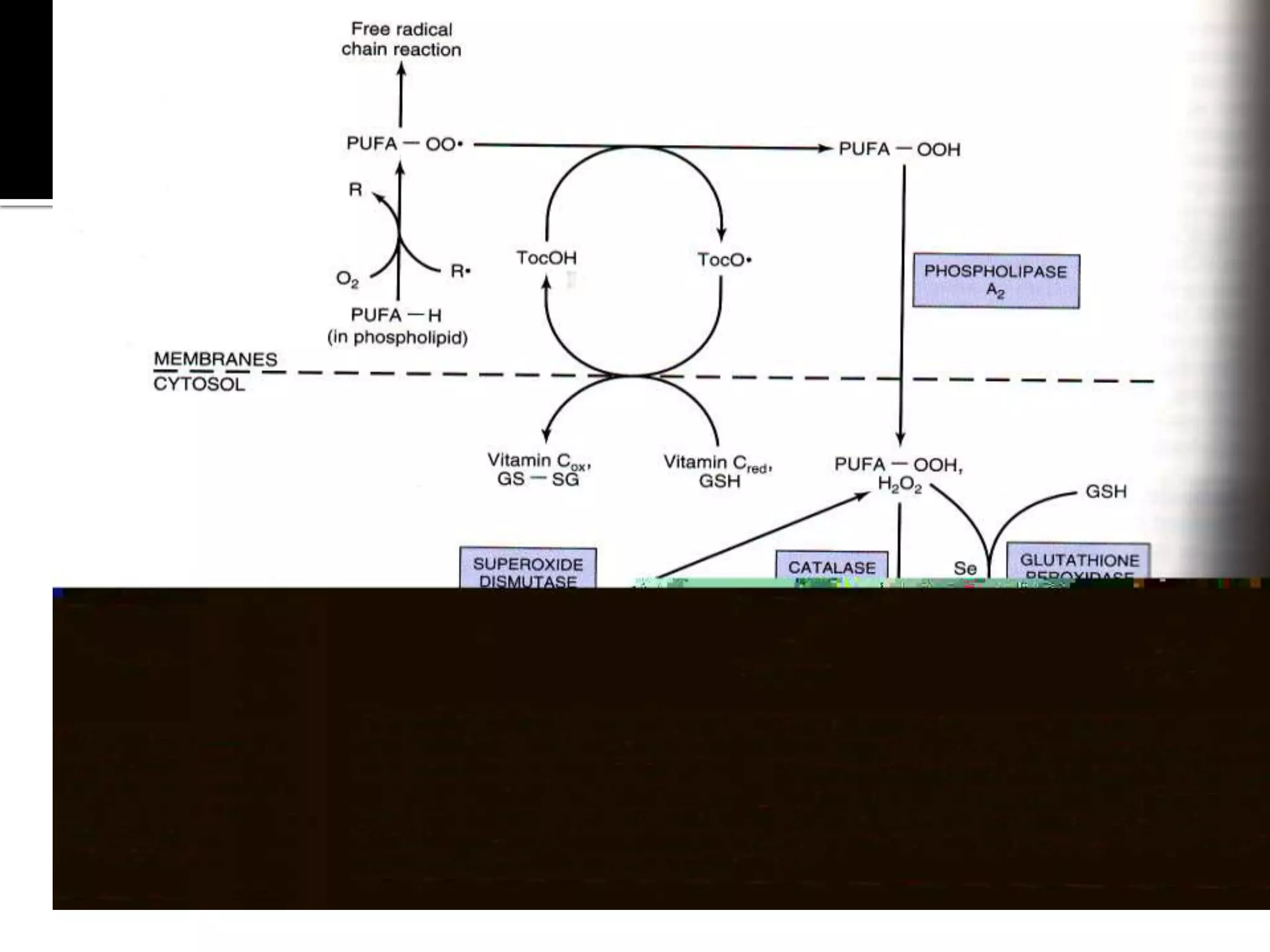
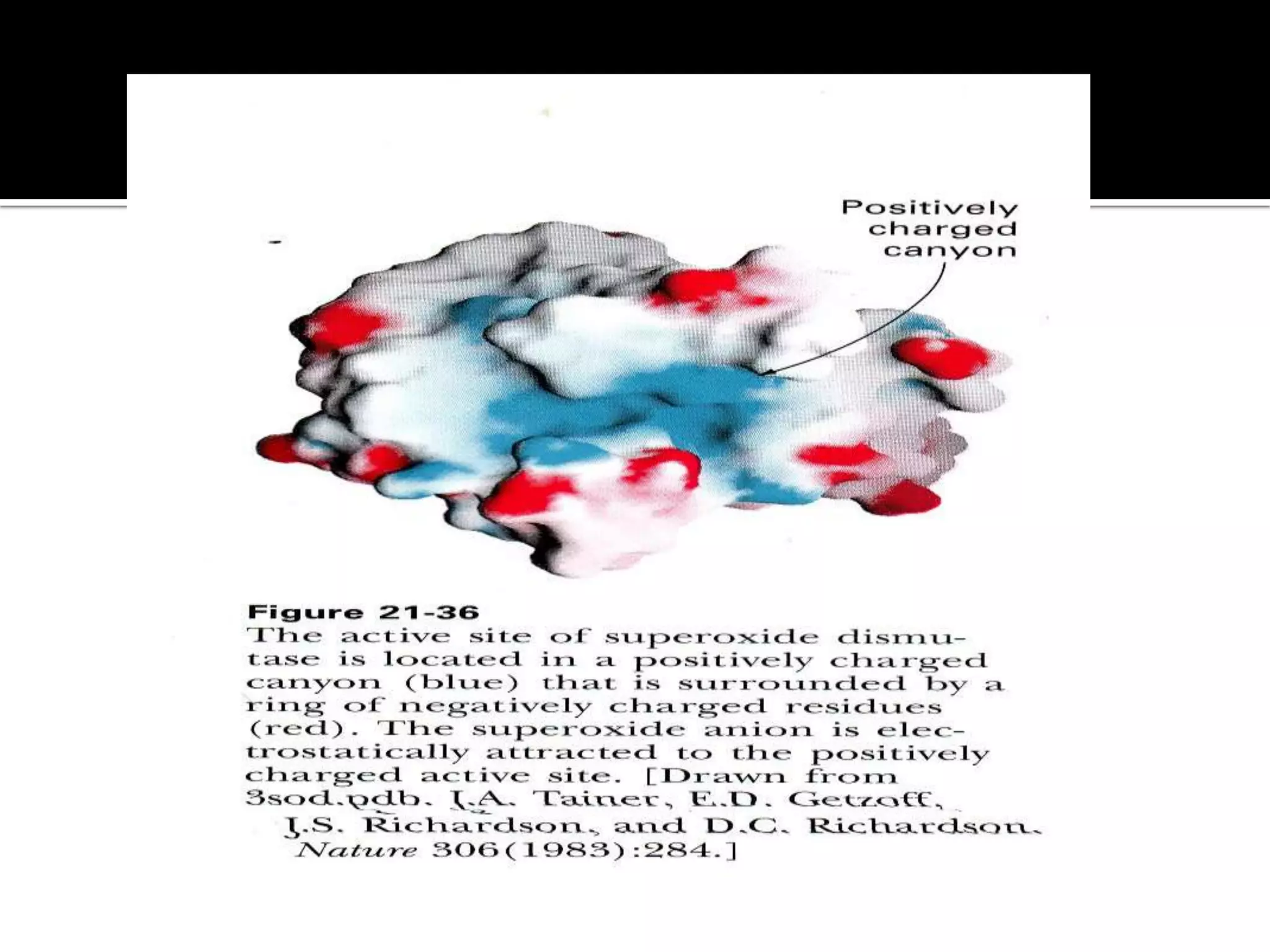
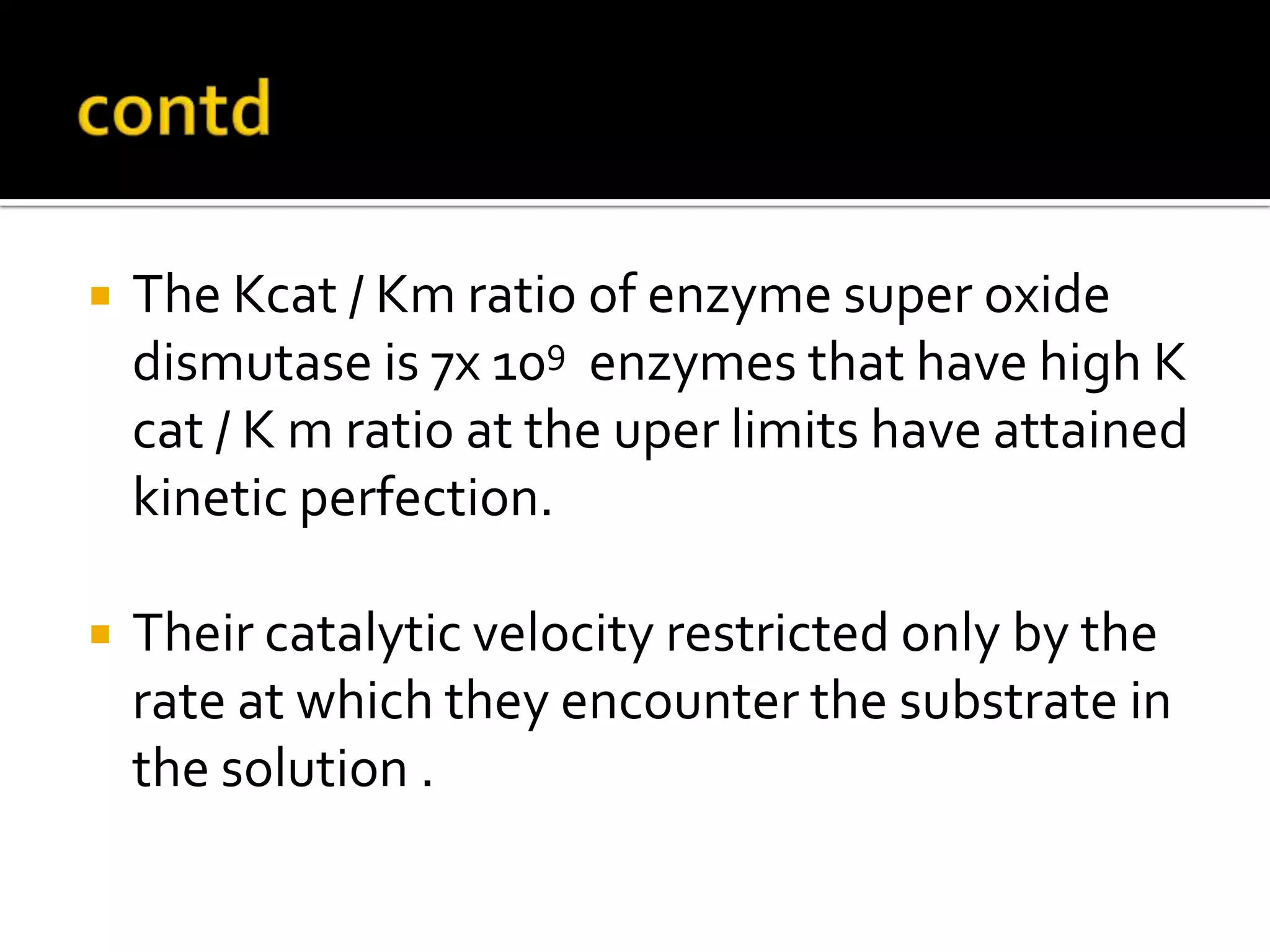
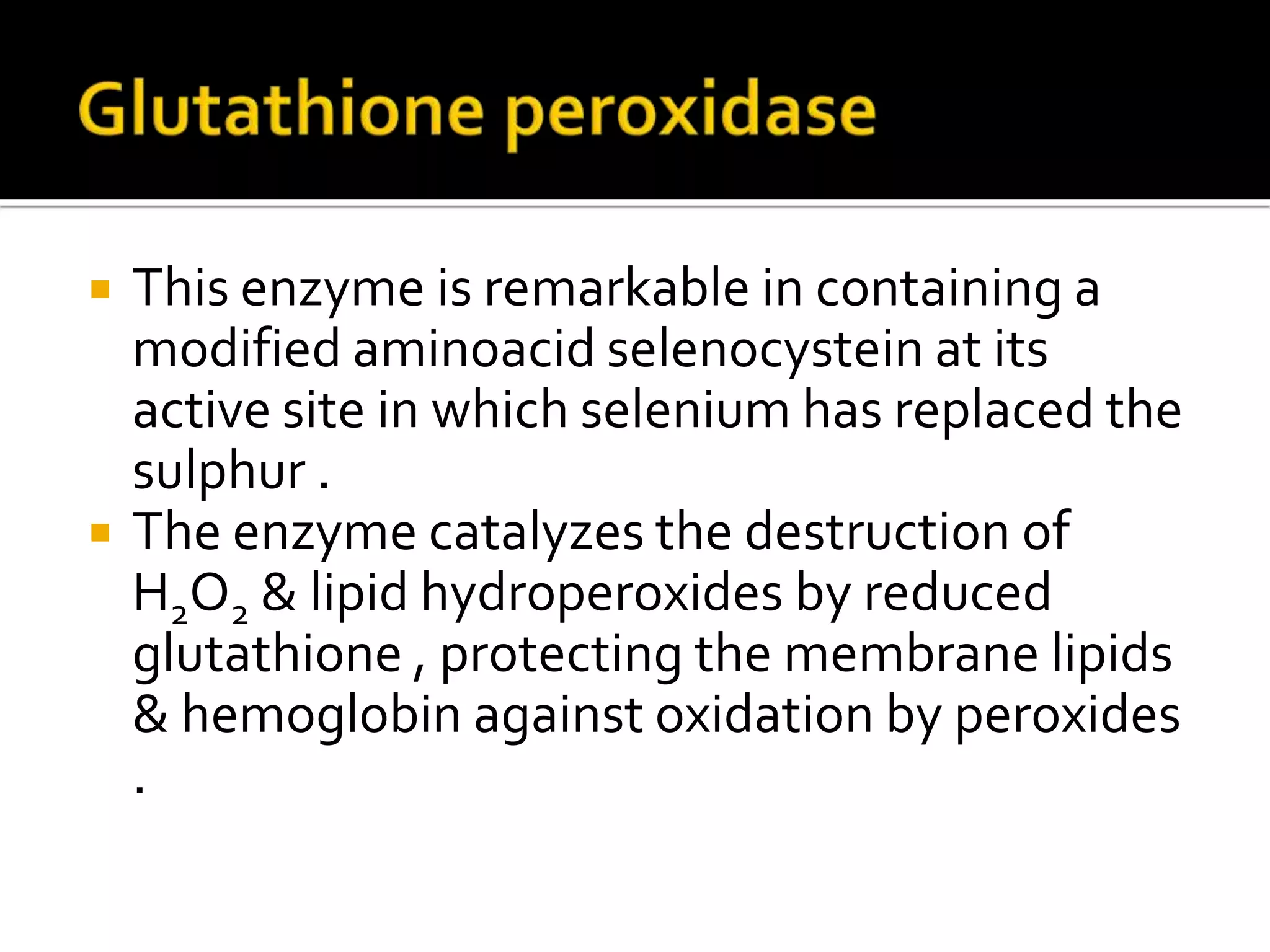
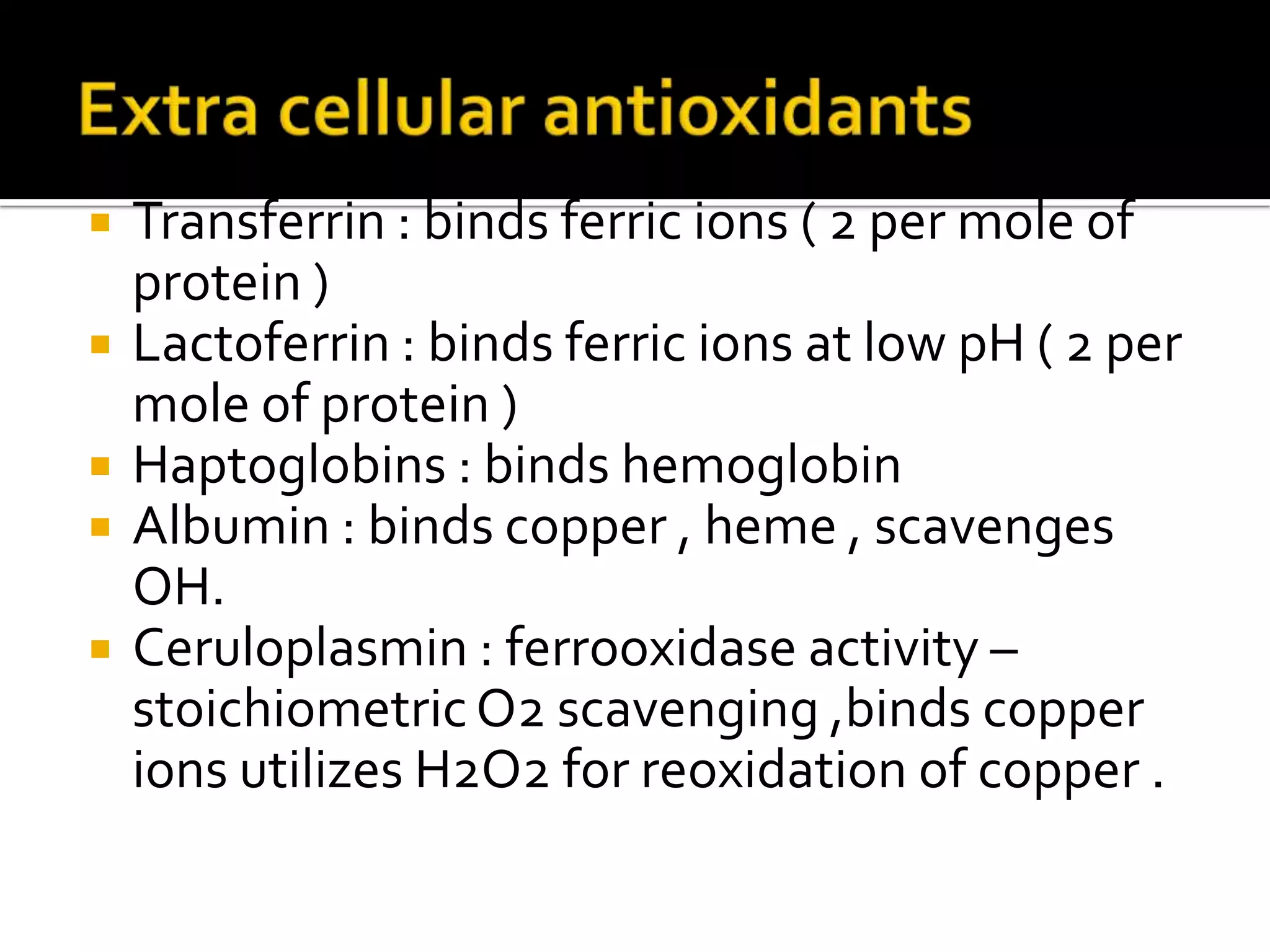
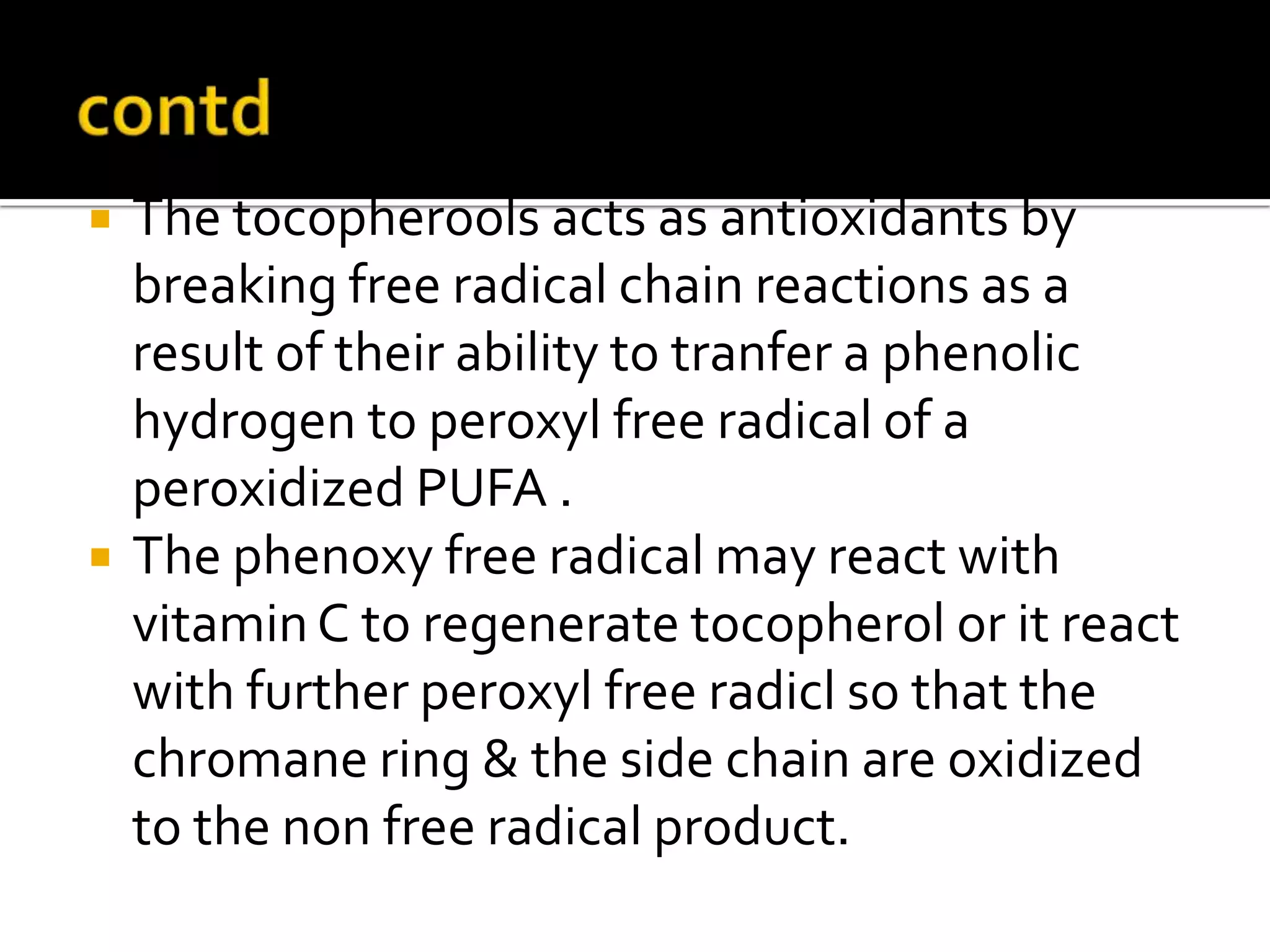
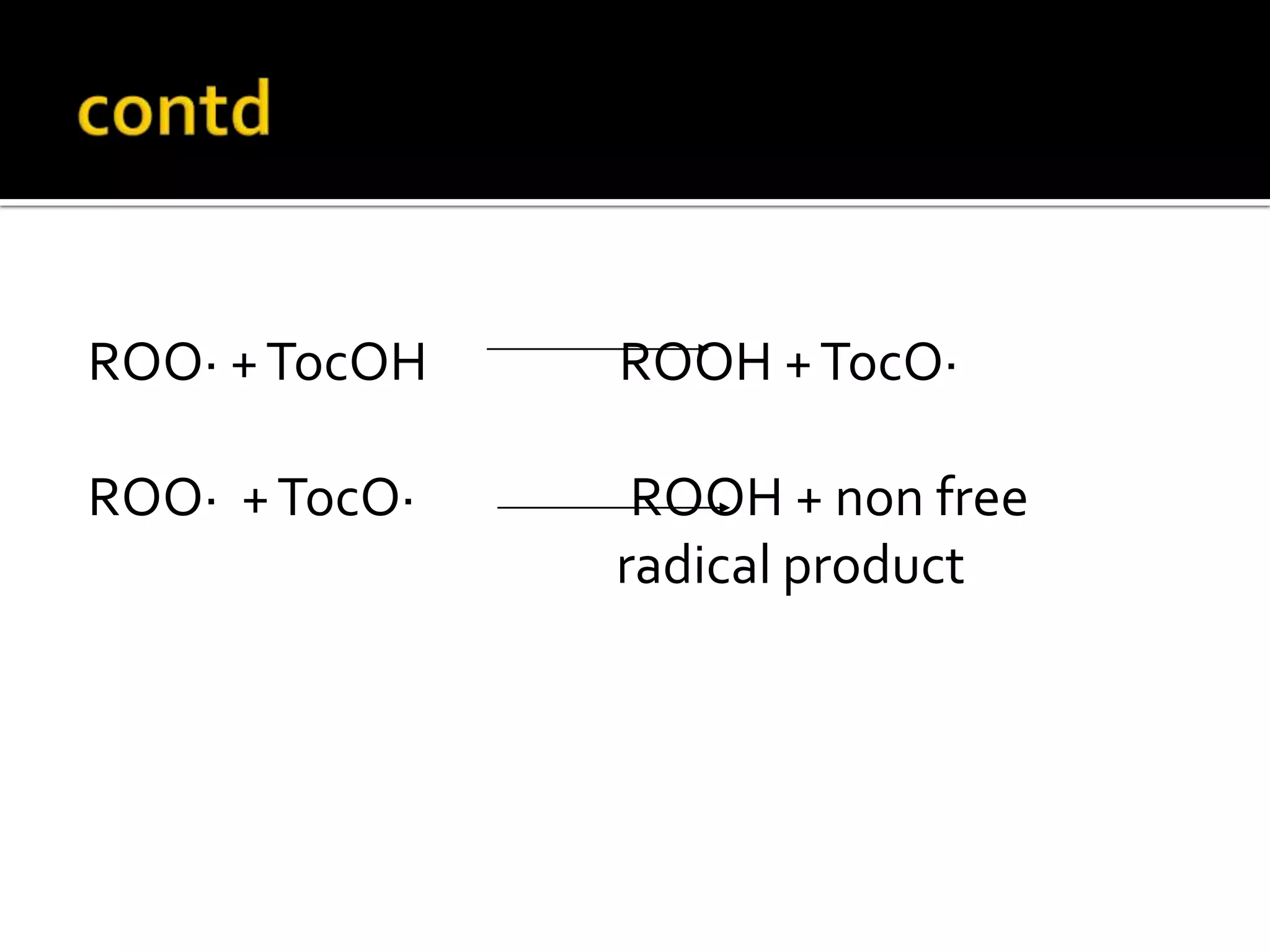
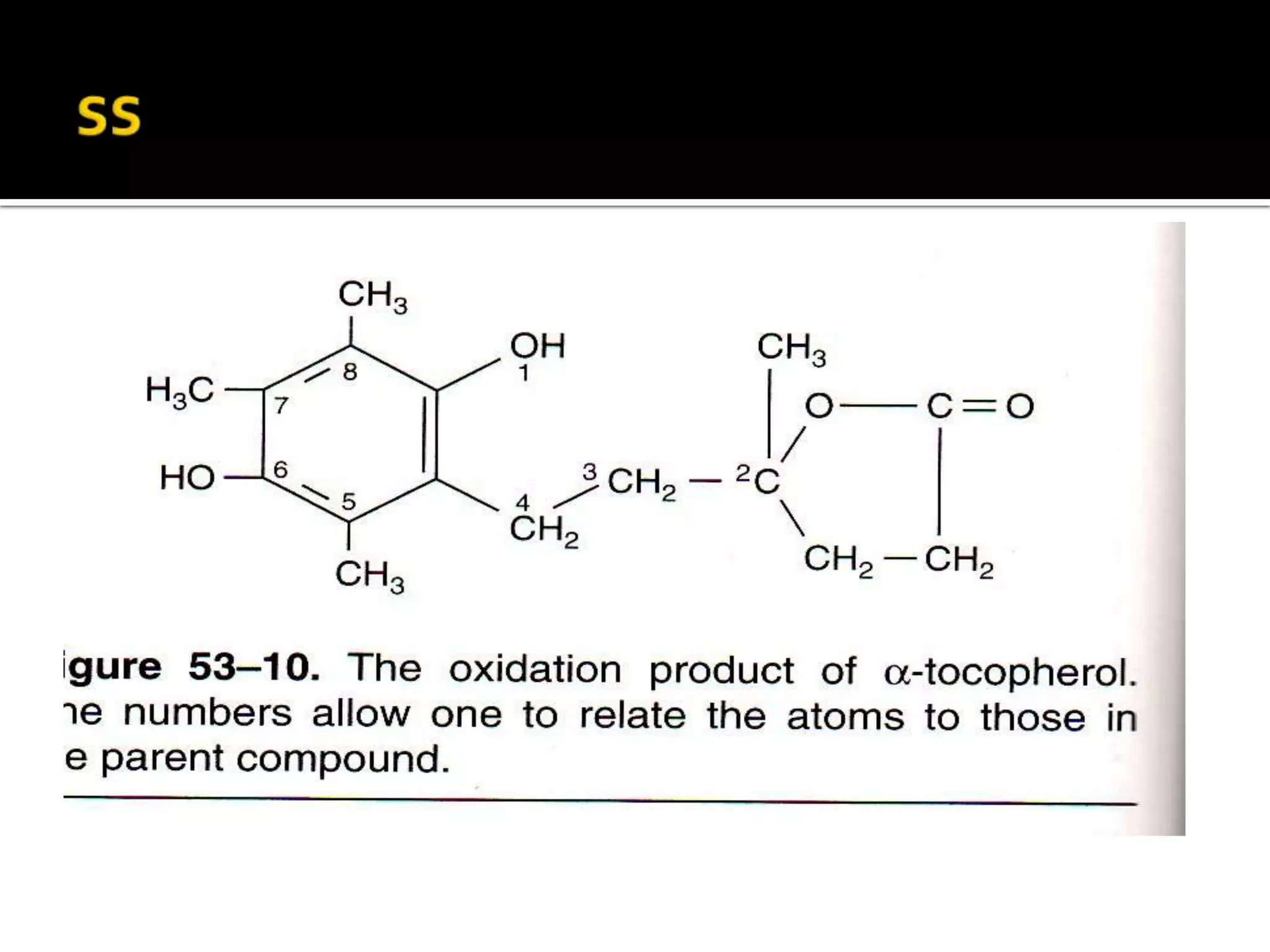
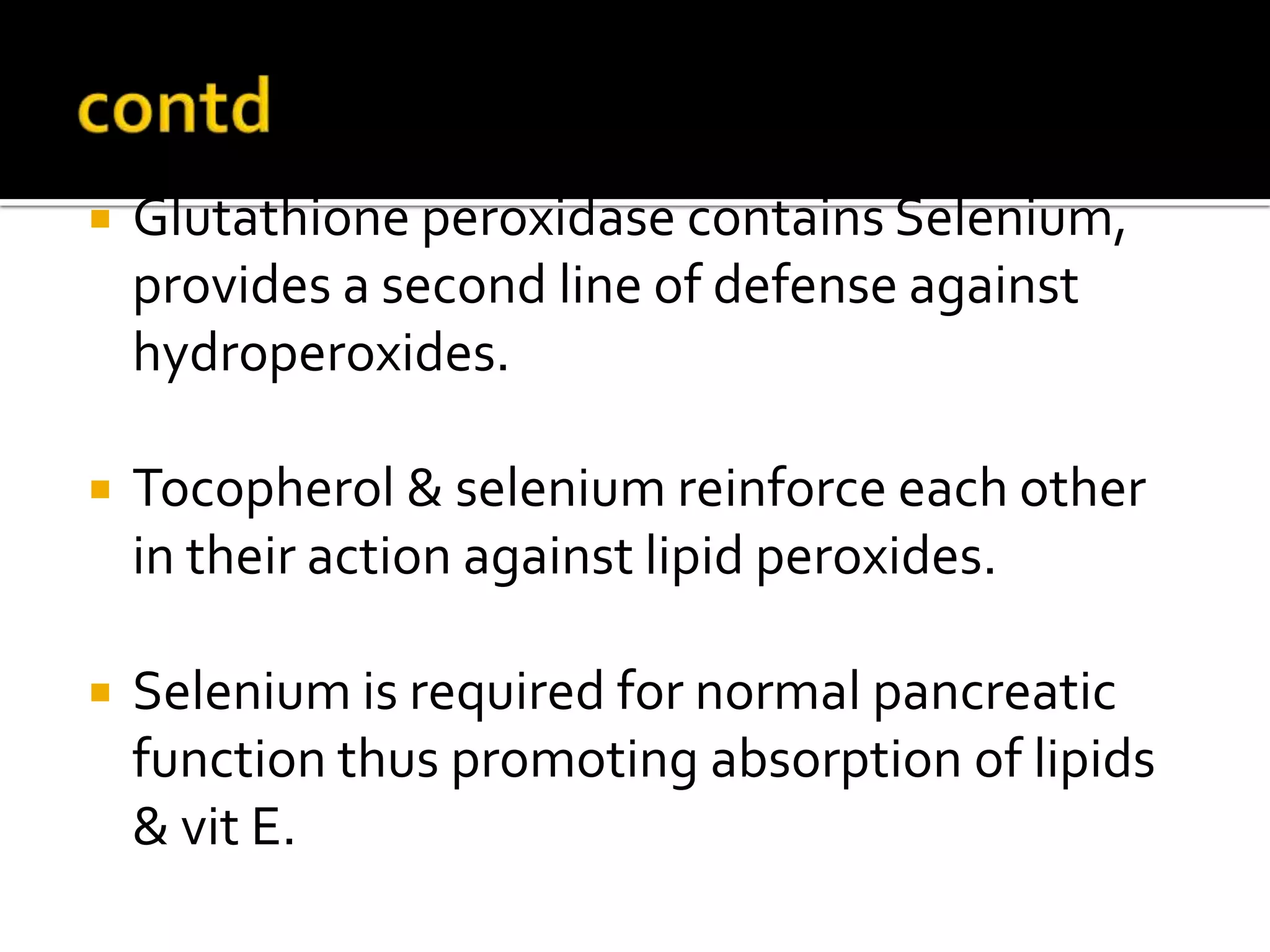
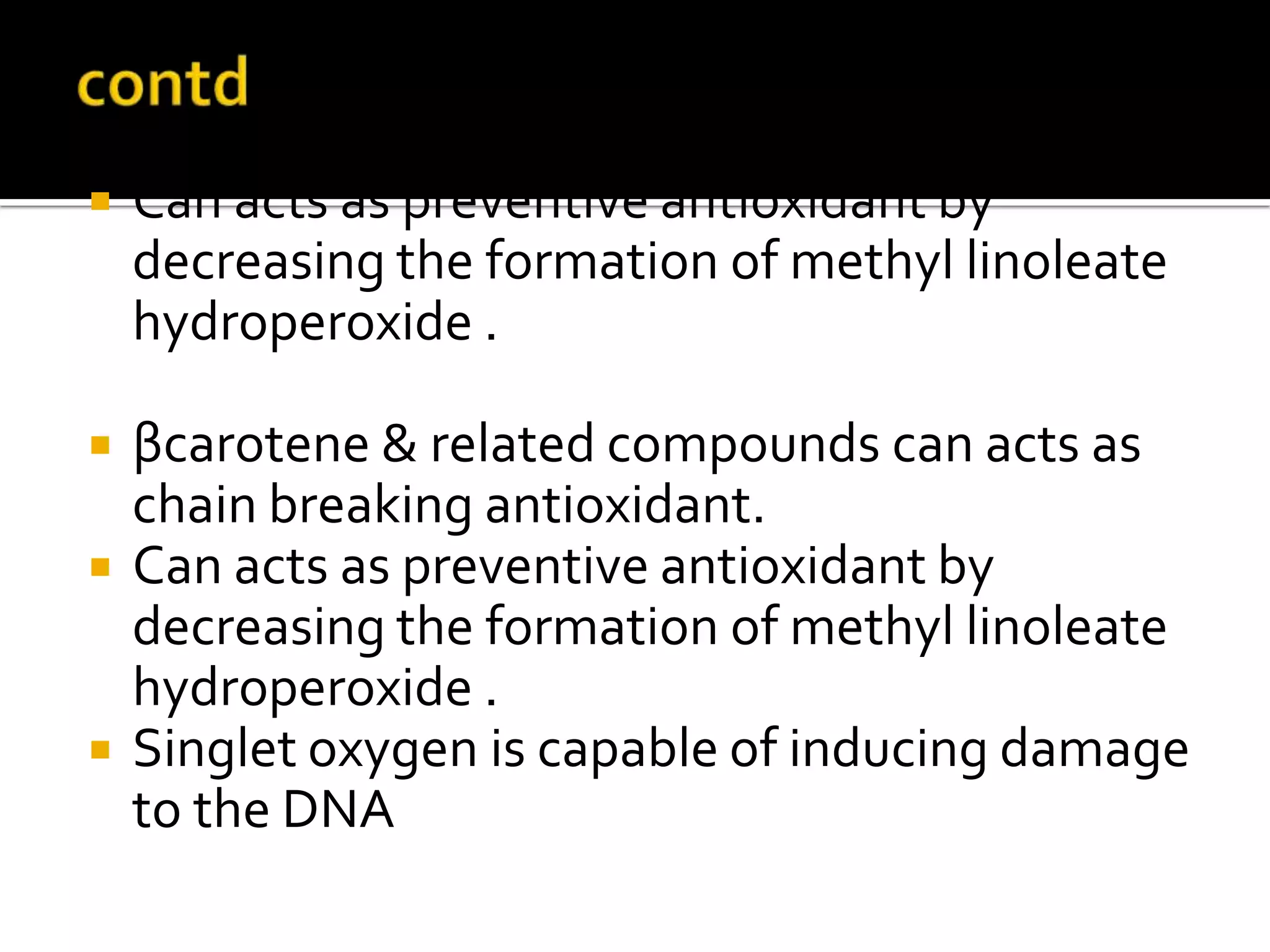
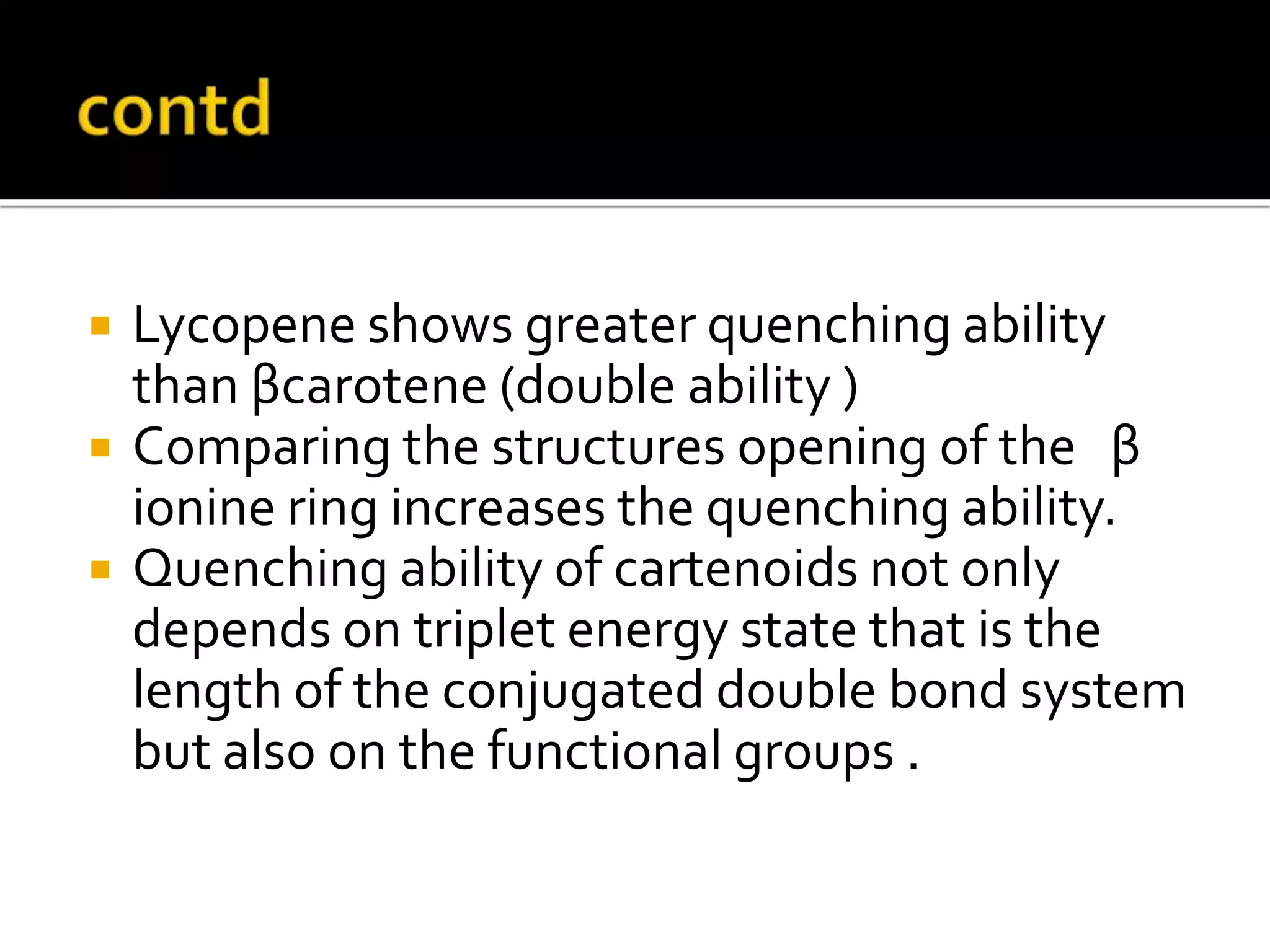
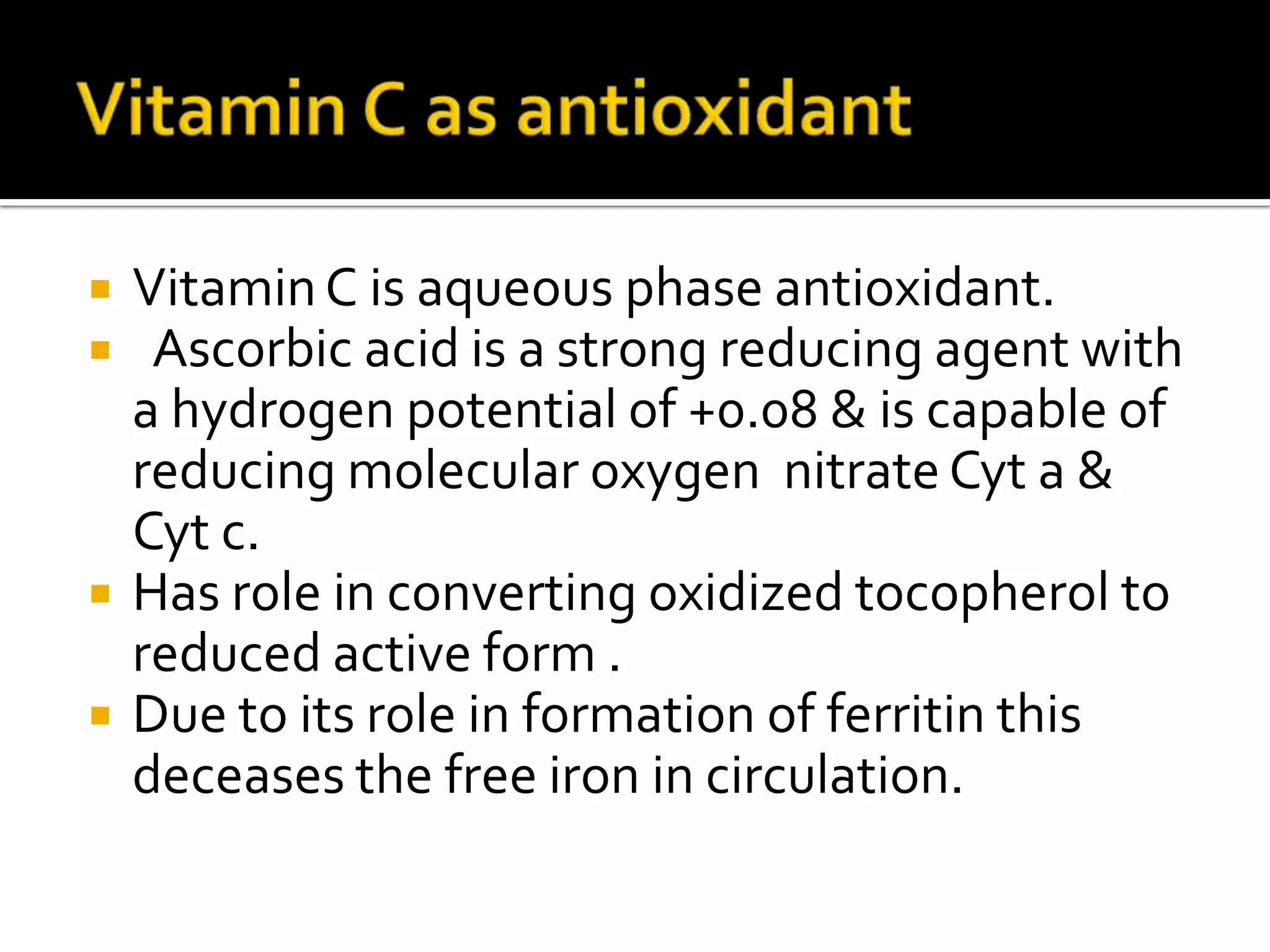
Free radicals are molecules with unpaired electrons that are highly reactive. They are generated through normal metabolic processes in the body and can cause damage. The body has antioxidant defenses against free radicals including enzymes like superoxide dismutase, catalase, and glutathione peroxidase which neutralize reactive oxygen species. Vitamins C and E also act as antioxidants to help prevent free radical damage to cells. While small amounts of free radicals occur naturally, excessive amounts from sources like pollution, smoking, or radiation can potentially cause harm if the body's defenses are overwhelmed.