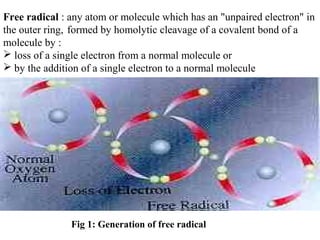
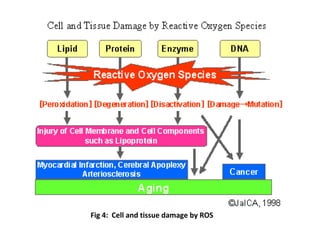
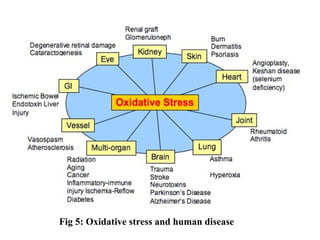
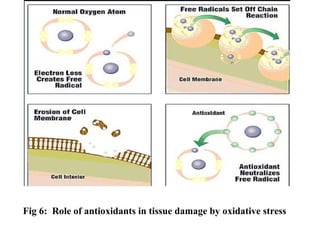
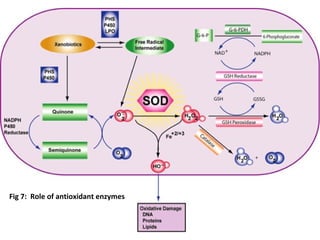
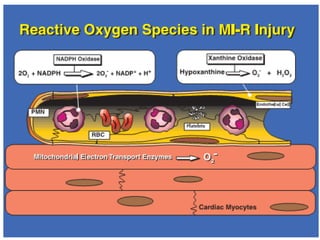
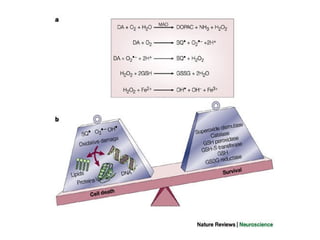
1. Reactive oxygen species (ROS) are generated through normal metabolic processes and can cause cell damage. Antioxidants help prevent this damage by neutralizing free radicals.
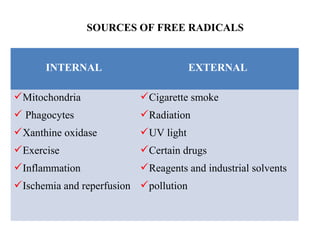
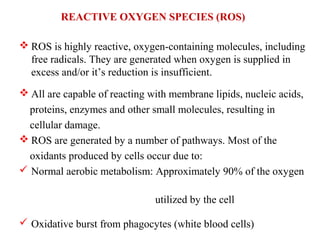
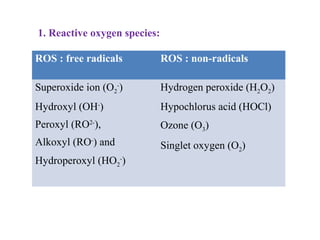
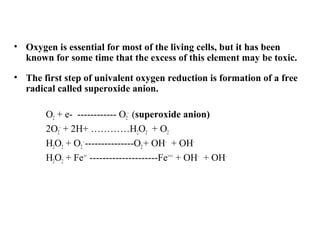
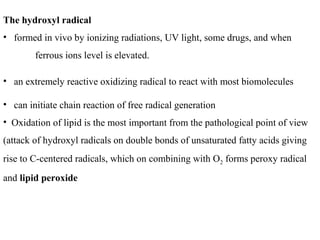
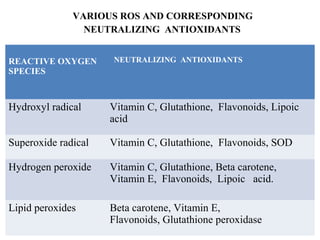
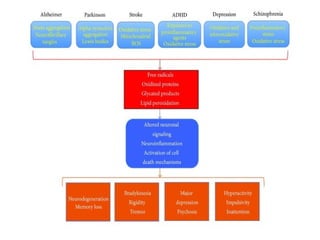
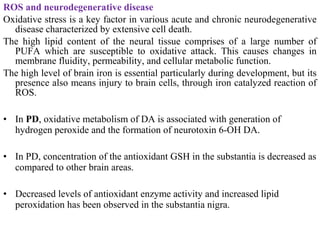
2. The document discusses various sources of ROS like mitochondria and inflammation, examples of ROS like superoxide and hydroxyl radicals, and the cell damage they can cause.
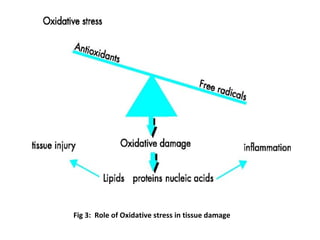
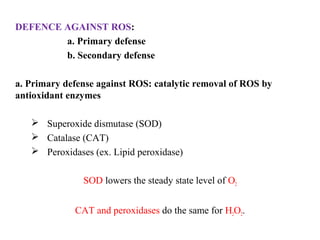
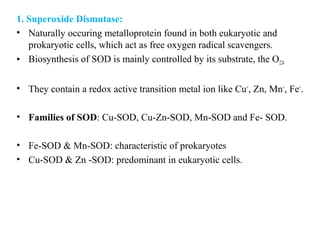
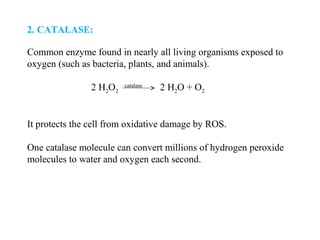
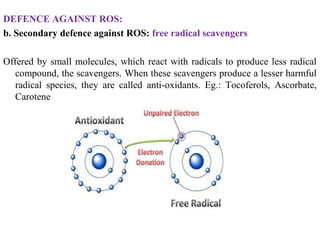
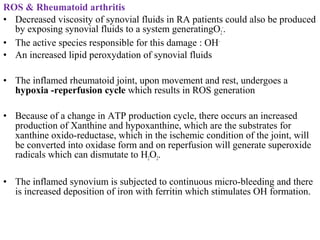
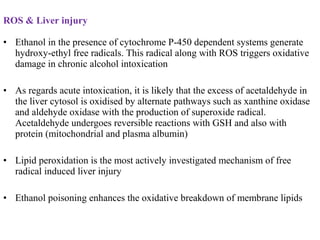
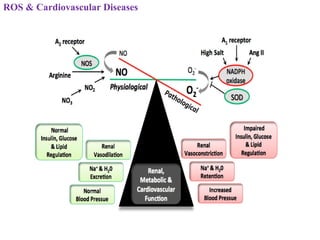
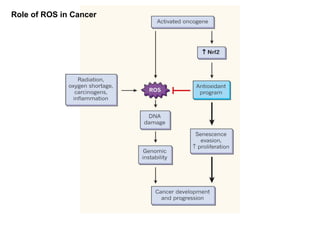
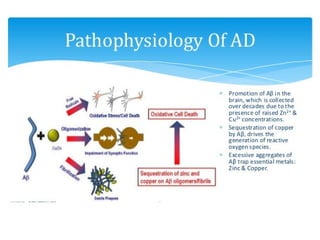
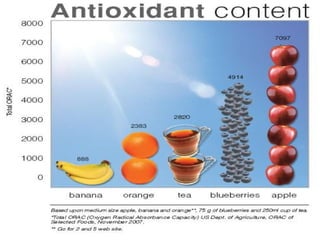
3. It also outlines the body's natural antioxidant protection systems including enzymes like superoxide dismutase and catalase, as well as dietary and plant-derived antioxidants. When antioxidant levels are insufficient to deal with ROS, oxidative stress can lead to diseases.