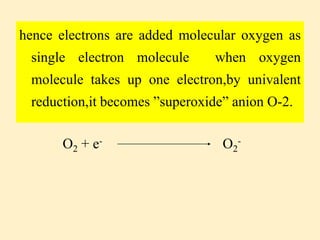
Aerobic organisms continuously produce reactive free radicals through respiration, metabolism and phagocytosis. Approximately 1-2% of oxygen consumed is converted into superoxide radicals by the respiratory chain, one of the main sources of free radicals in cells. While oxygen is necessary for life, its partial reduction can produce reactive oxygen species (ROS) that damage living systems. The body has multiple antioxidant defenses to combat ROS, including superoxide dismutase, catalase, glutathione peroxidase and vitamin C, which help convert ROS into less reactive species and protect biomolecules from oxidative damage.