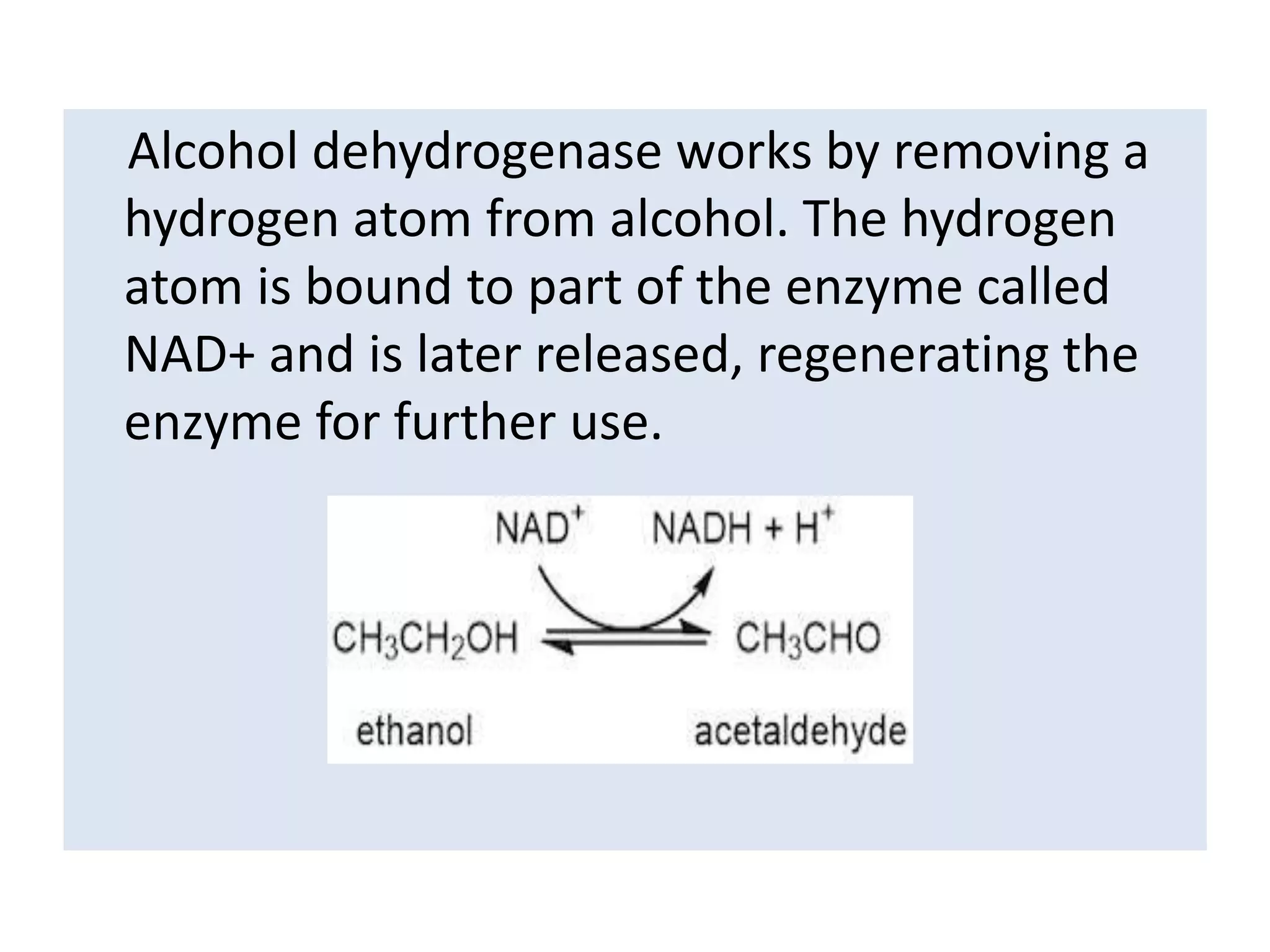
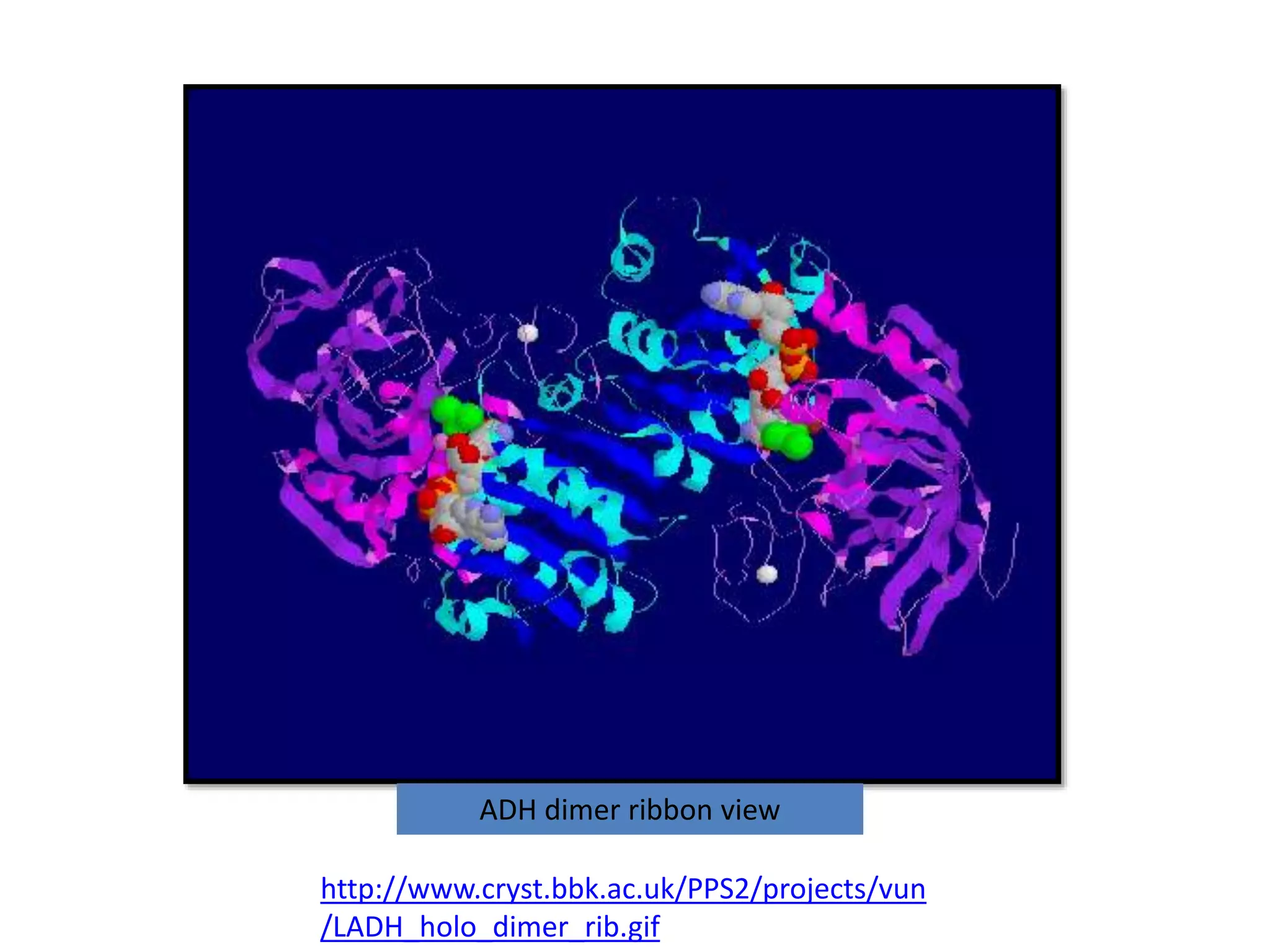
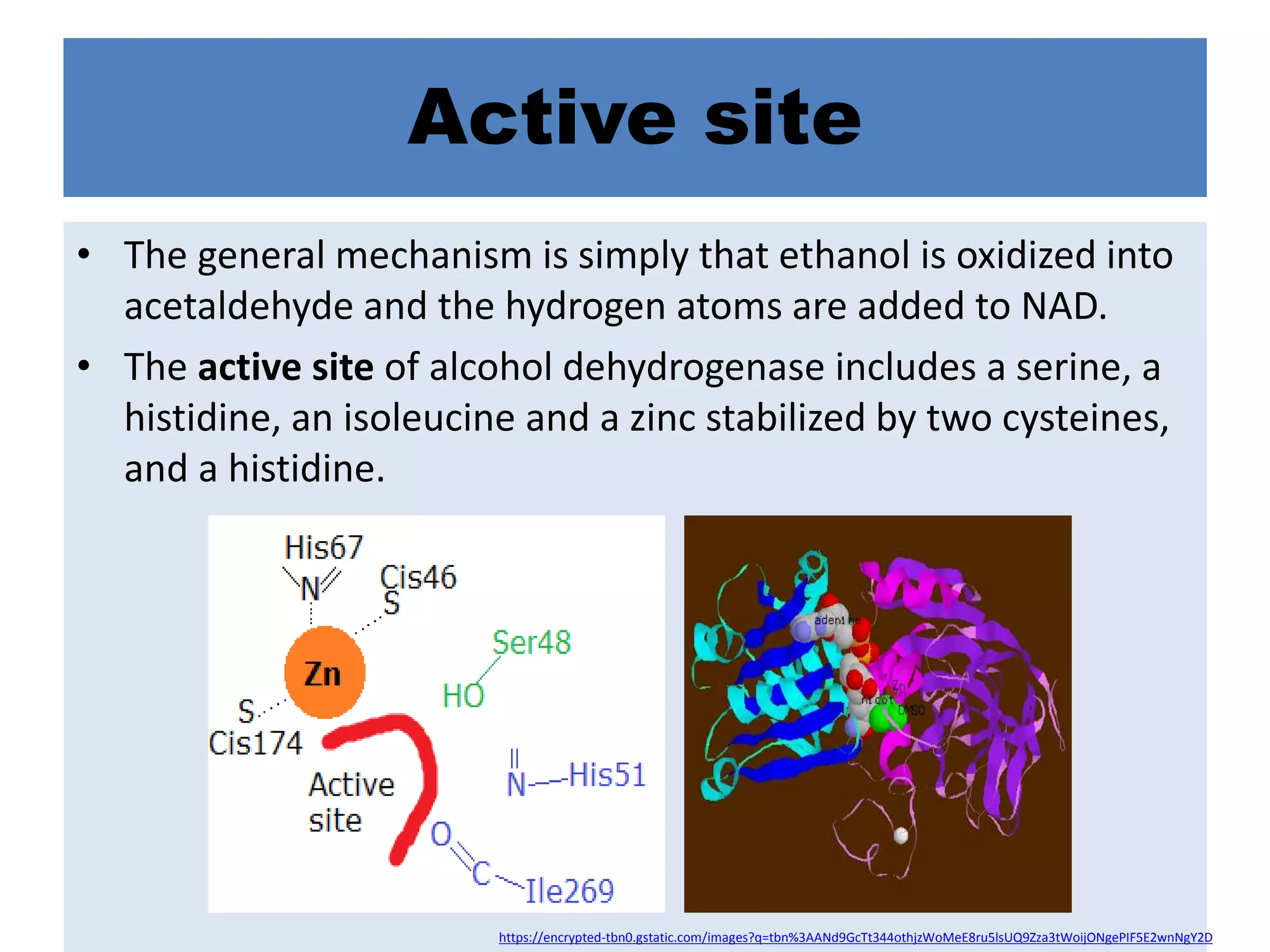
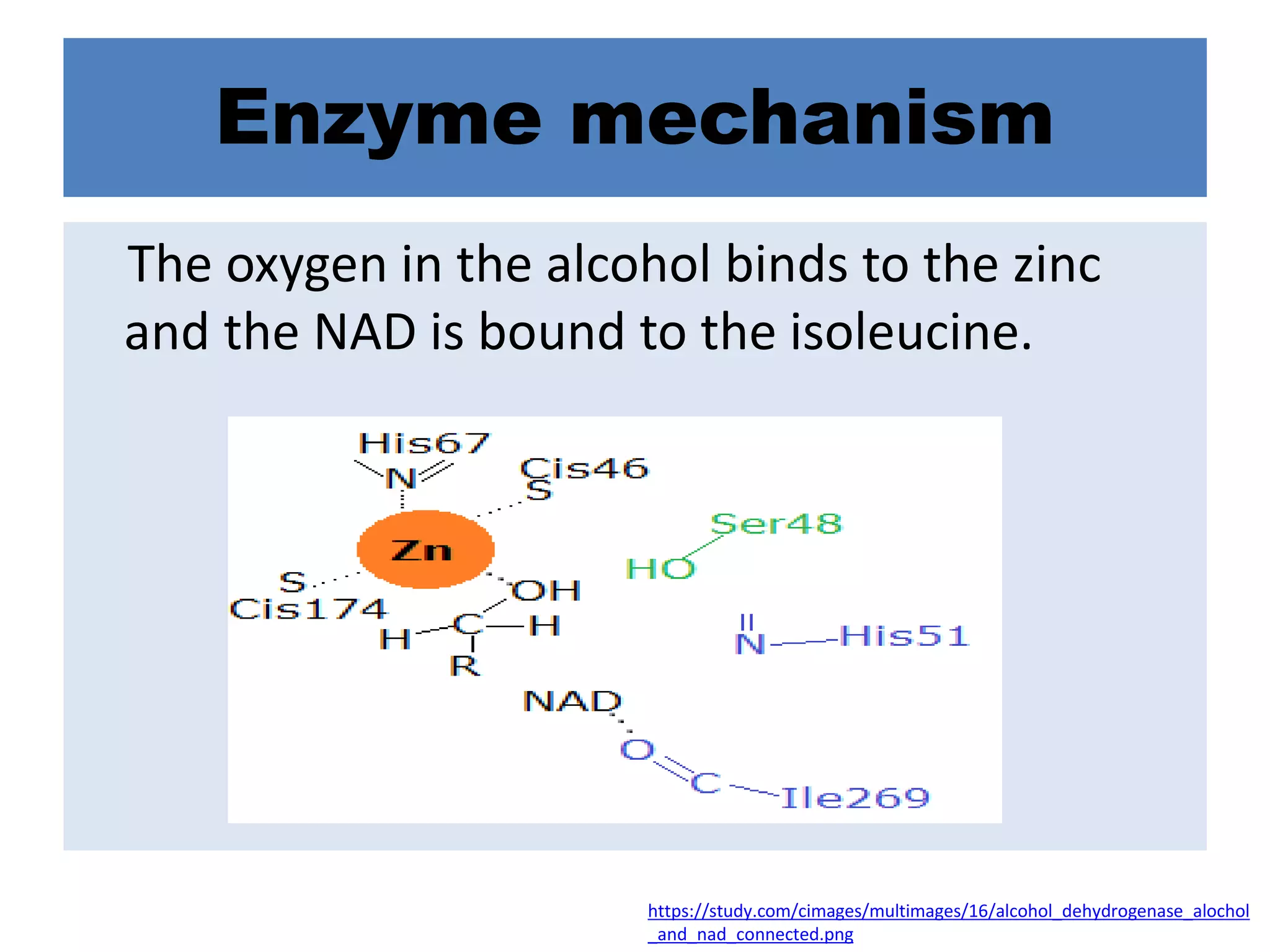
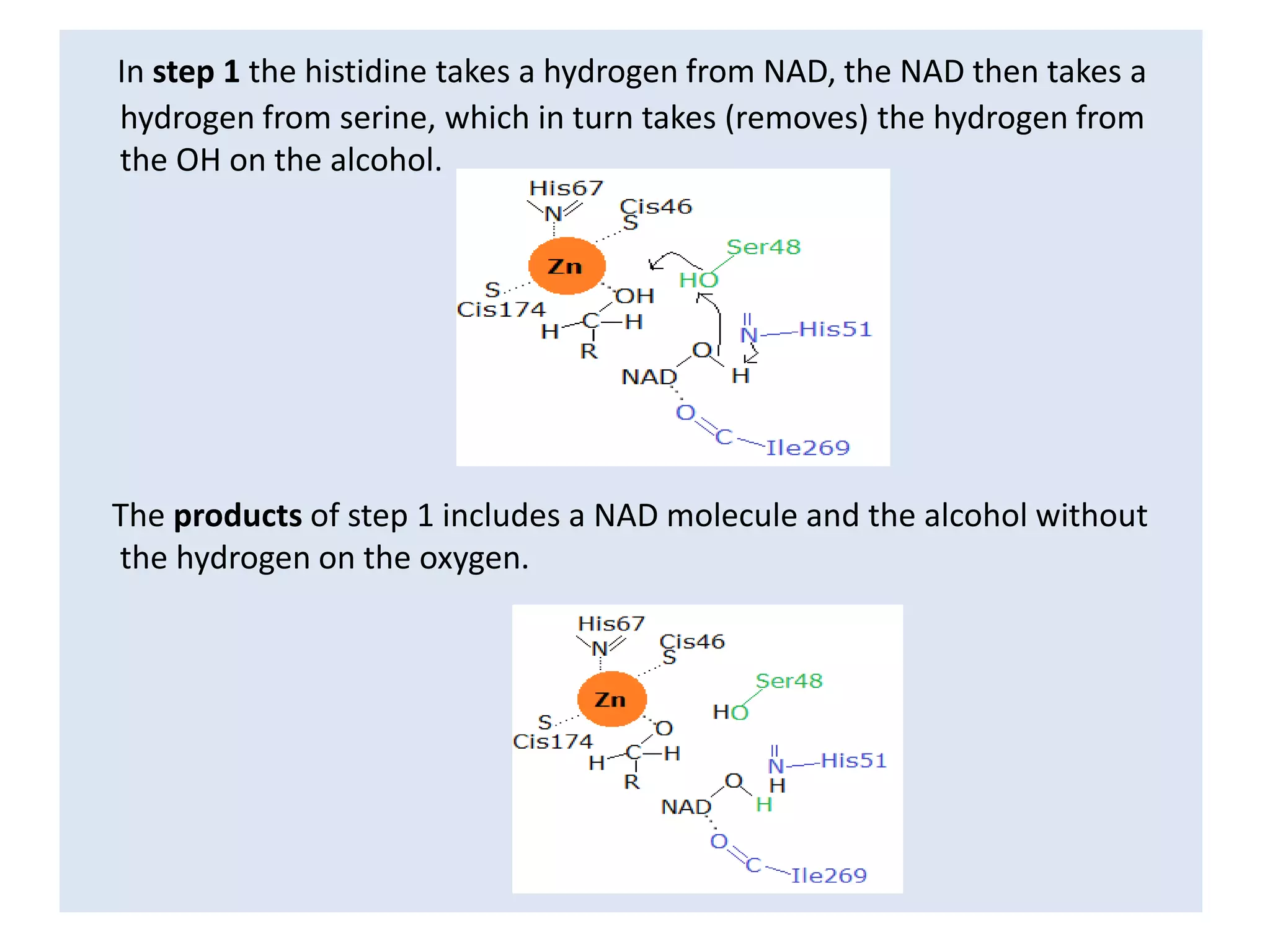
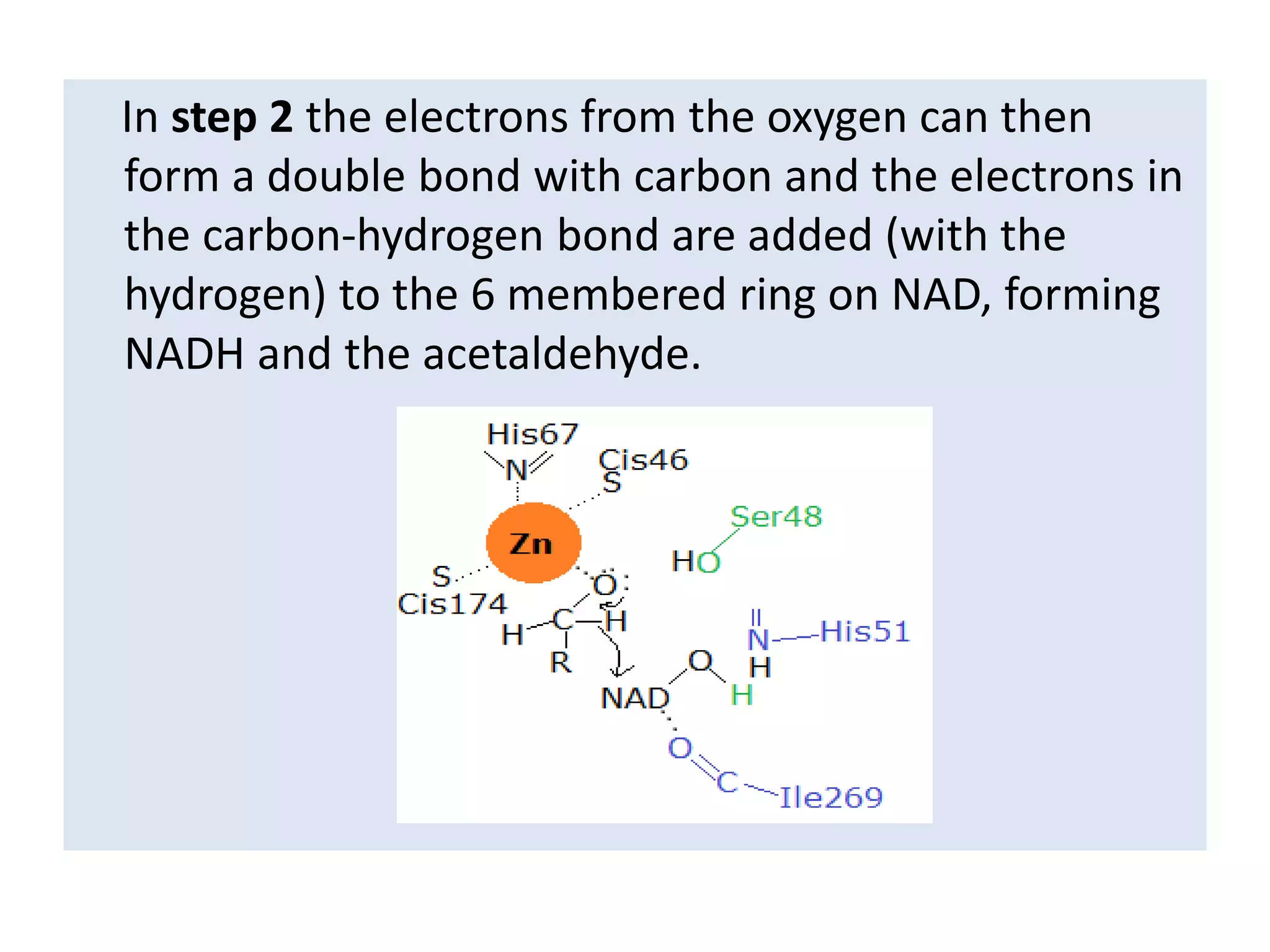
Alcohol dehydrogenase is an enzyme that converts alcohols to aldehydes or ketones through the reduction of NAD+ to NADH. It is a zinc-containing dimer found primarily in the liver and stomach lining. The enzyme removes a hydrogen atom from alcohol and transfers it to NAD+, converting the alcohol to an aldehyde and reducing NAD+ to NADH. It has two domains and uses a zinc ion and residues like serine and histidine in its catalytic site to facilitate this reaction through two steps. Competitive inhibitors like fomepizole are used to treat ethylene glycol or methanol poisoning by slowing their metabolism to toxic products.