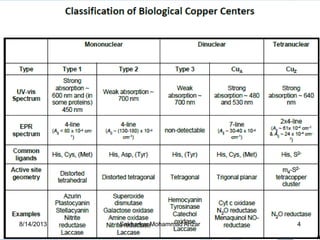
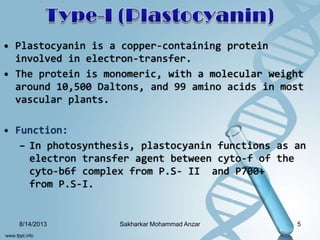
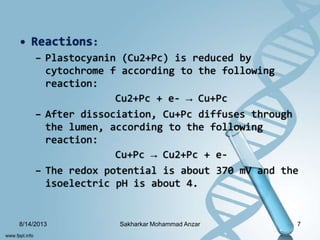
The document discusses several copper-containing proteins including plastocyanin, copper amine oxidase, hemocyanin, cytochrome c oxidase, tyrosinase, and superoxide dismutase. It describes their structures, catalytic functions, and roles in electron transfer reactions and oxidation processes in photosynthesis, respiration, and melanin production. Deficiencies and disorders related to defects in these copper proteins are also mentioned.