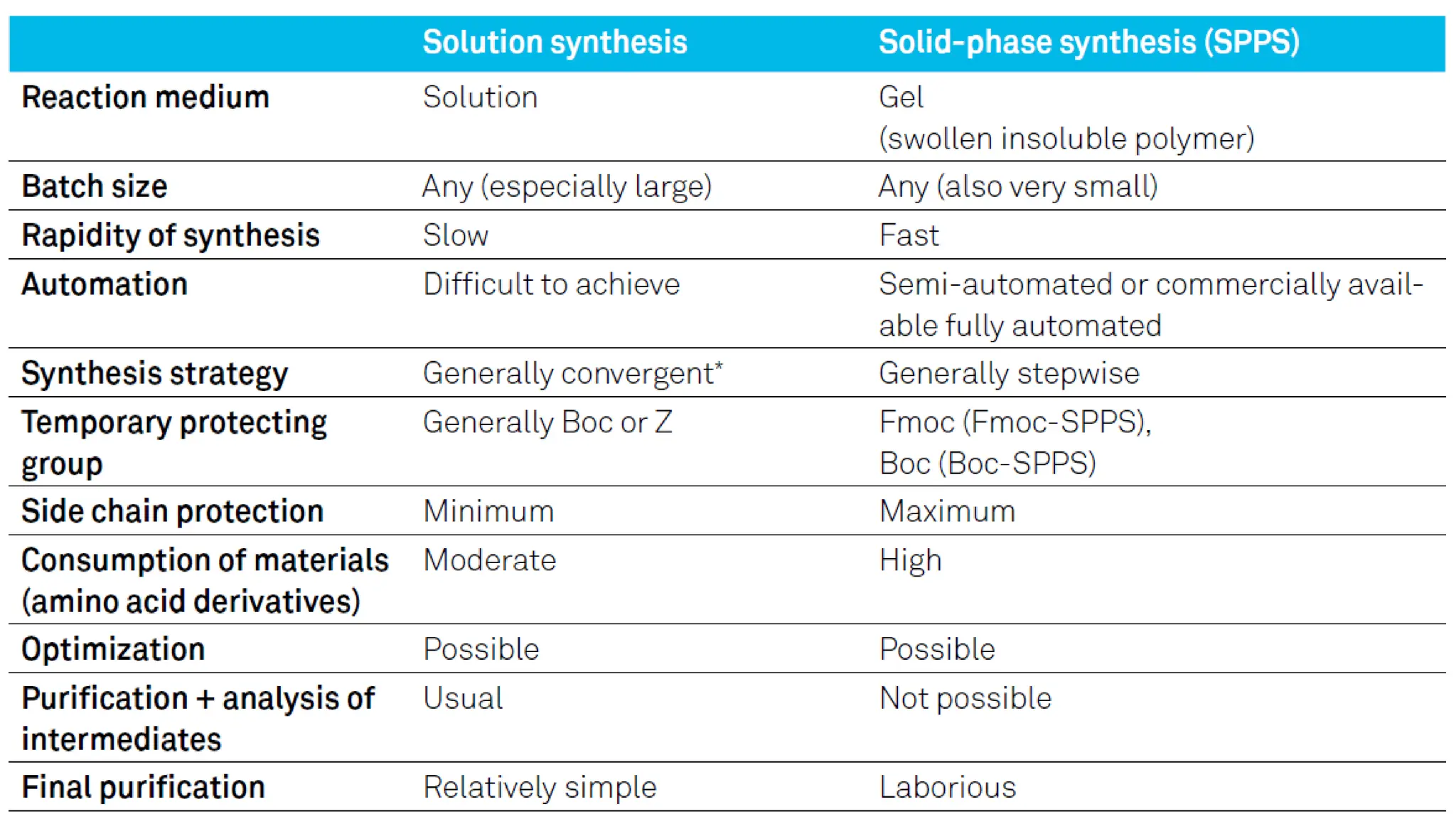
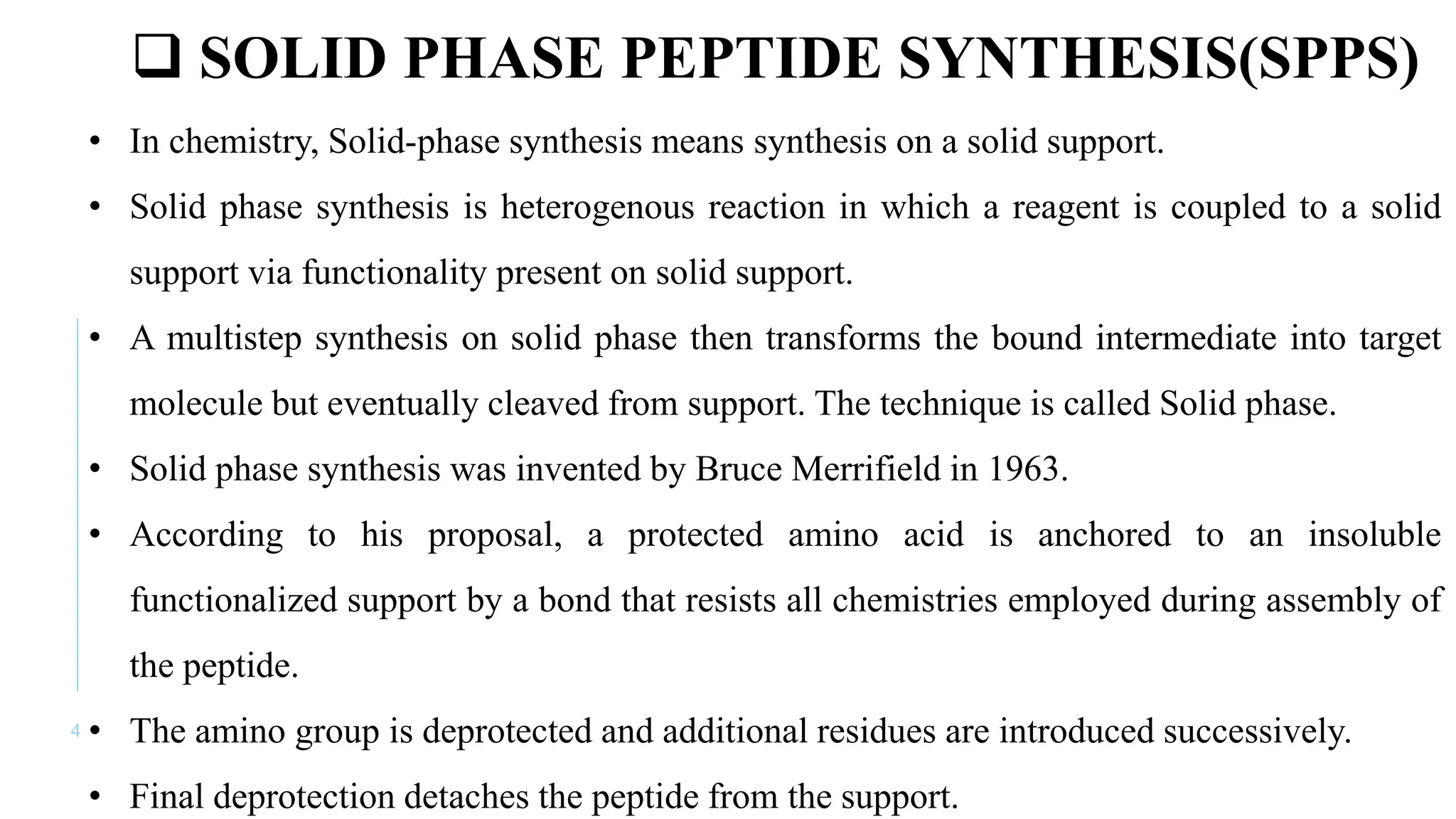
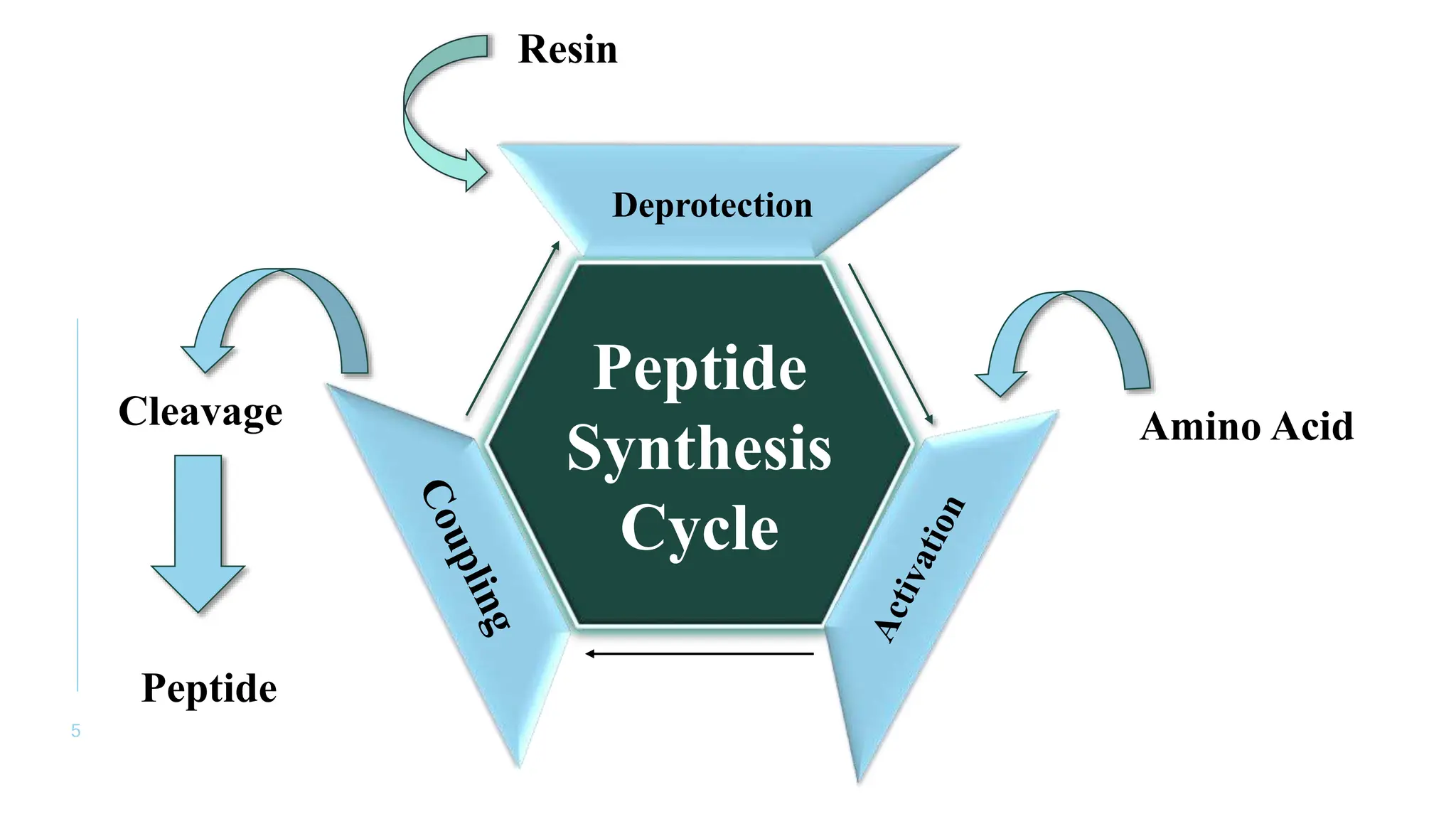
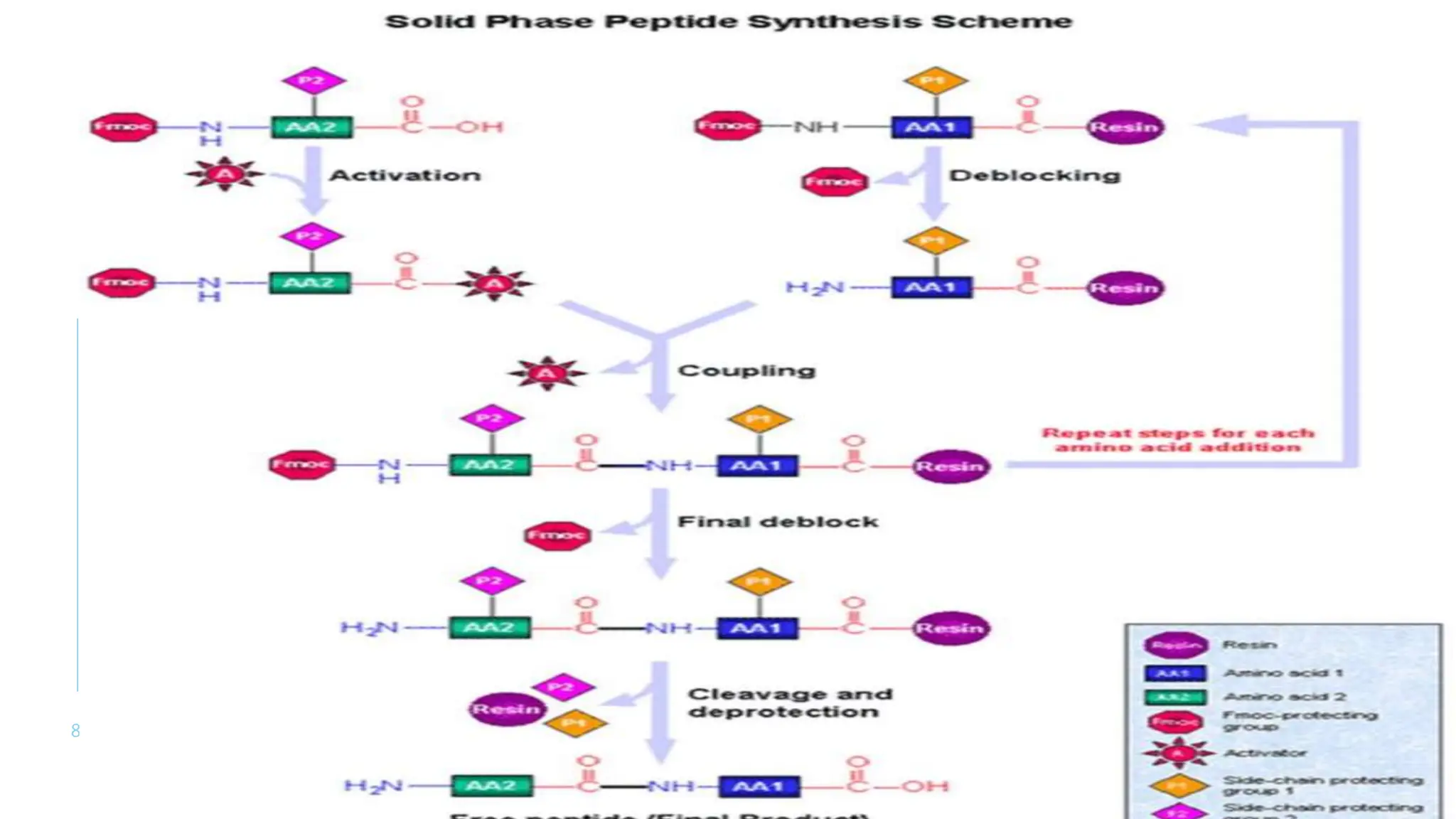
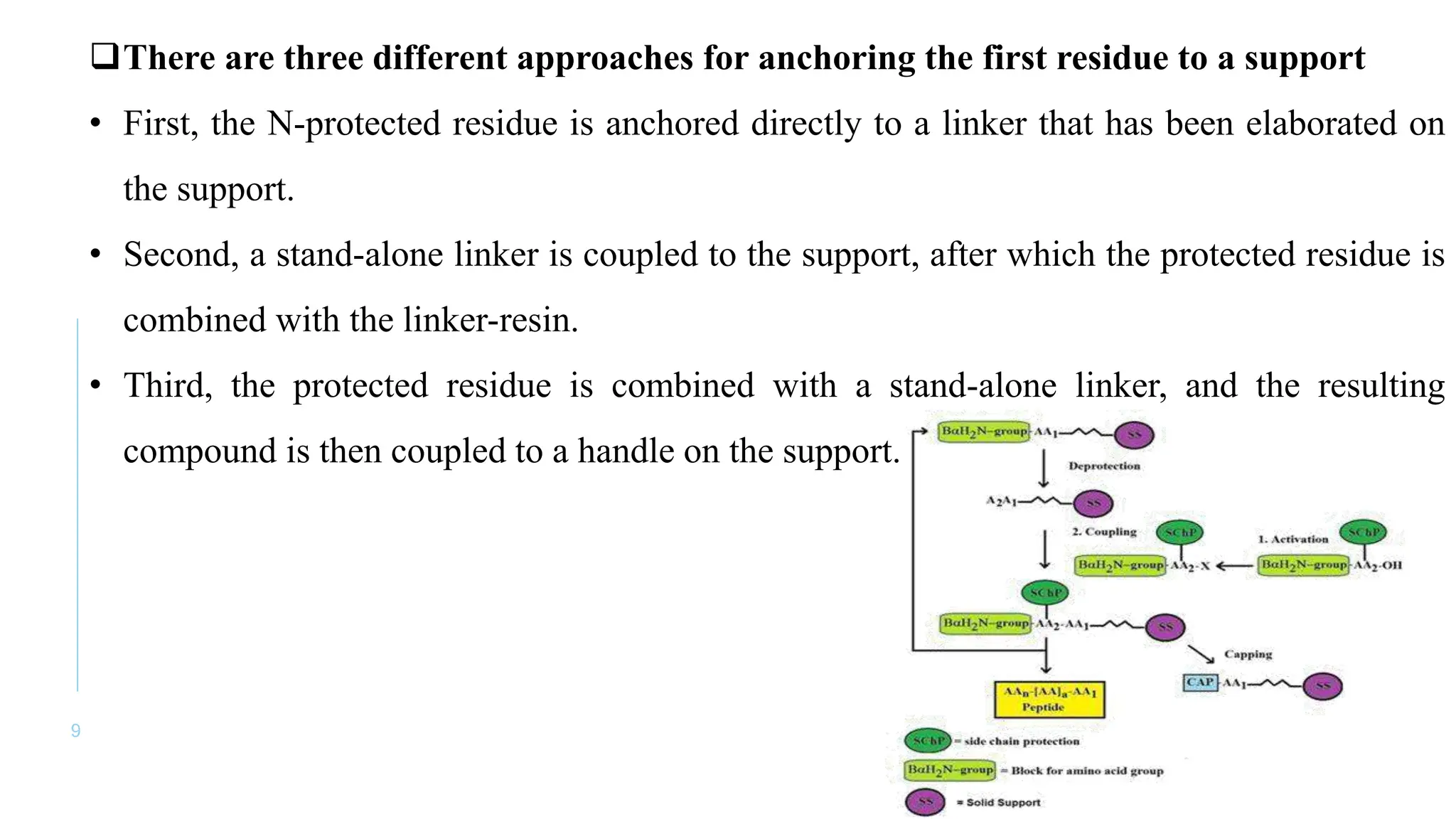
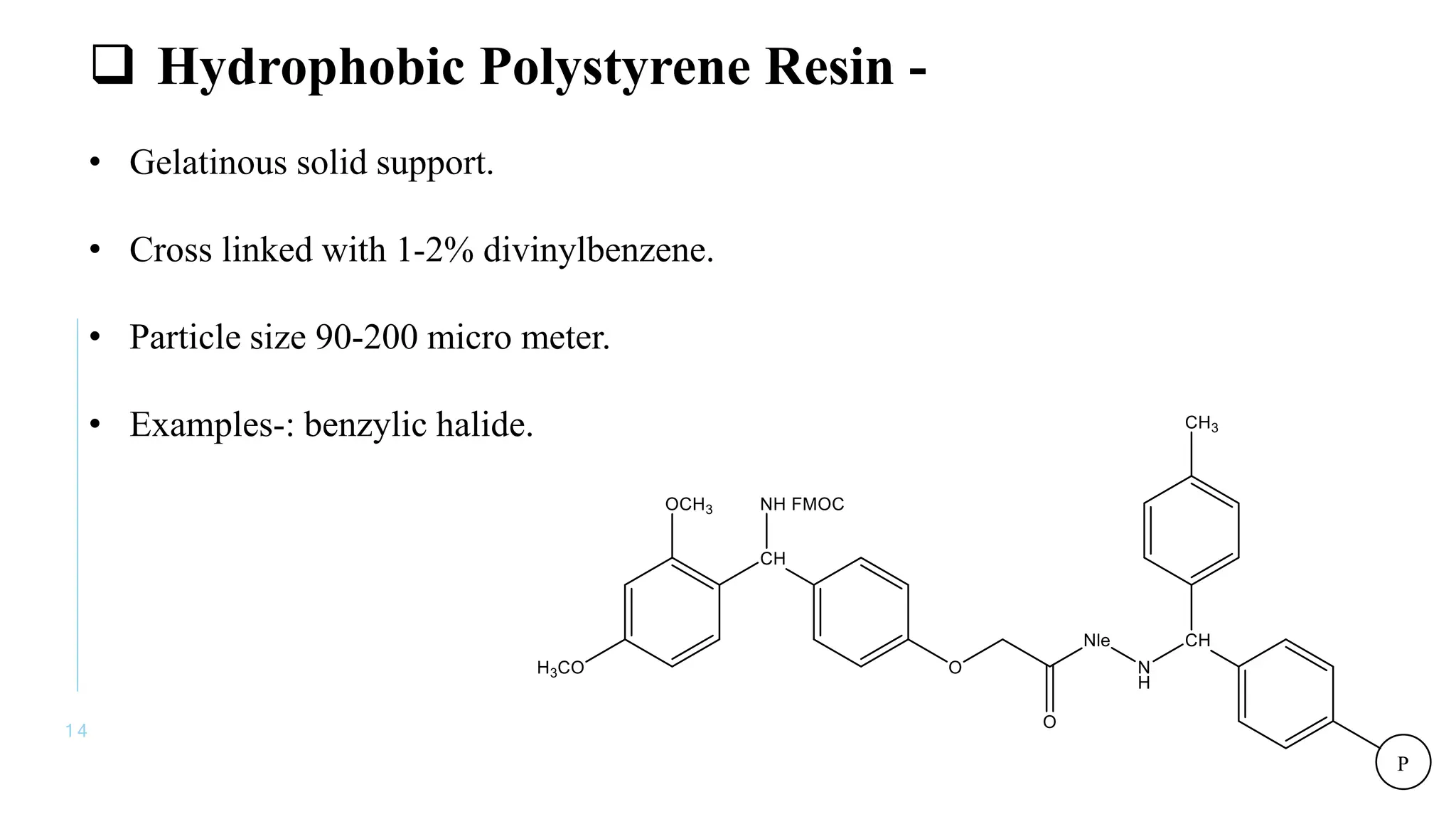
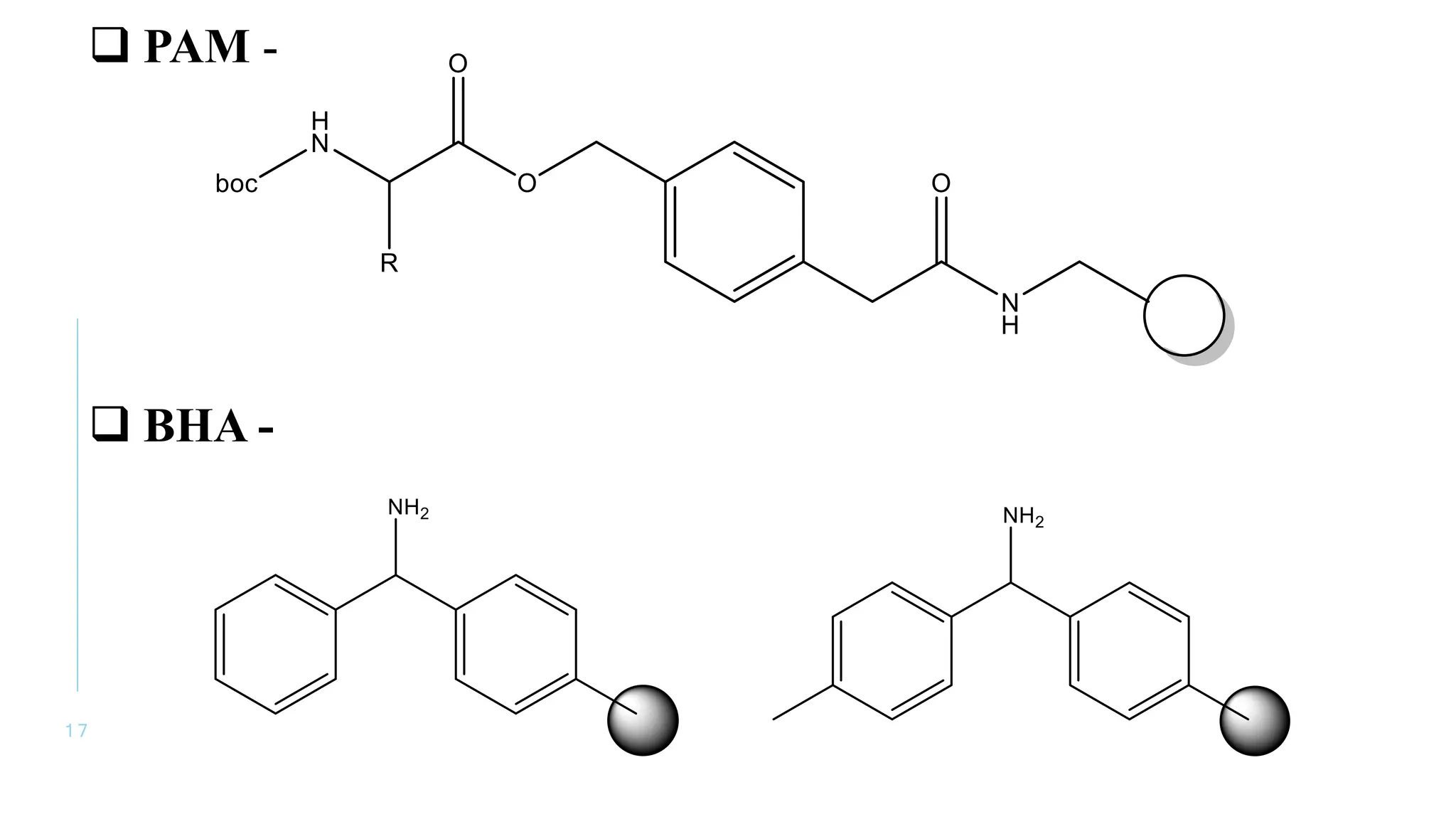
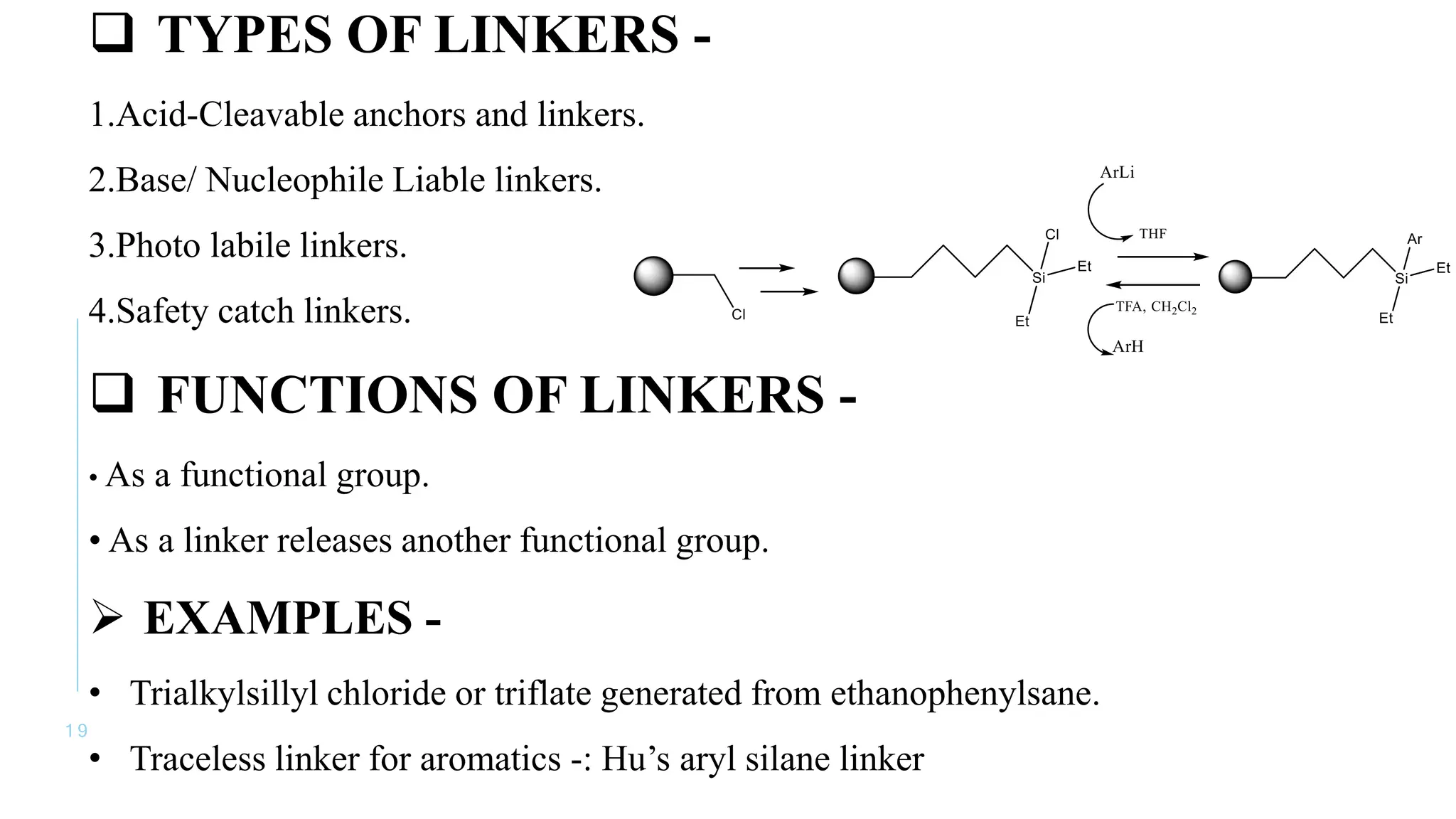
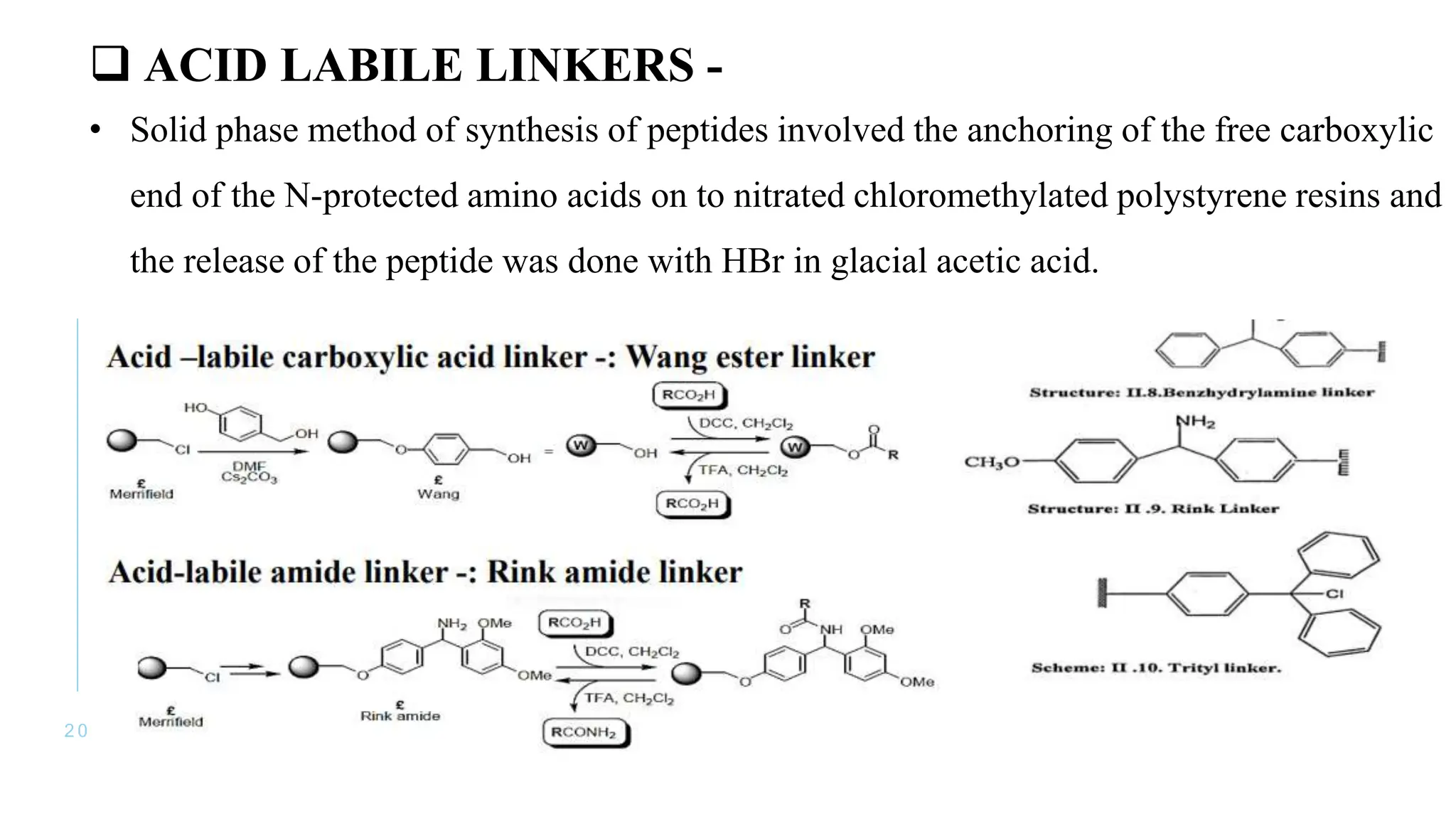
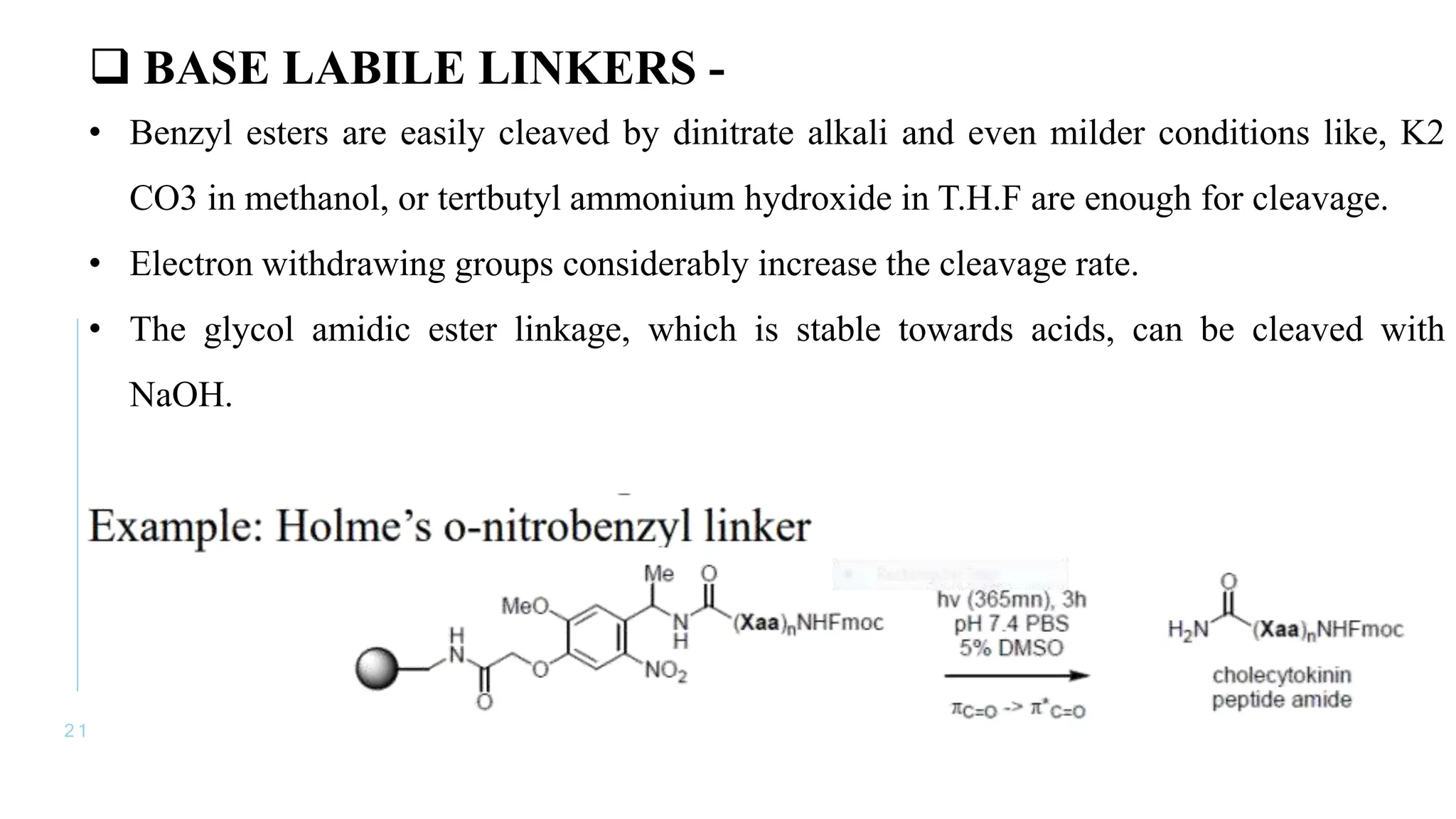
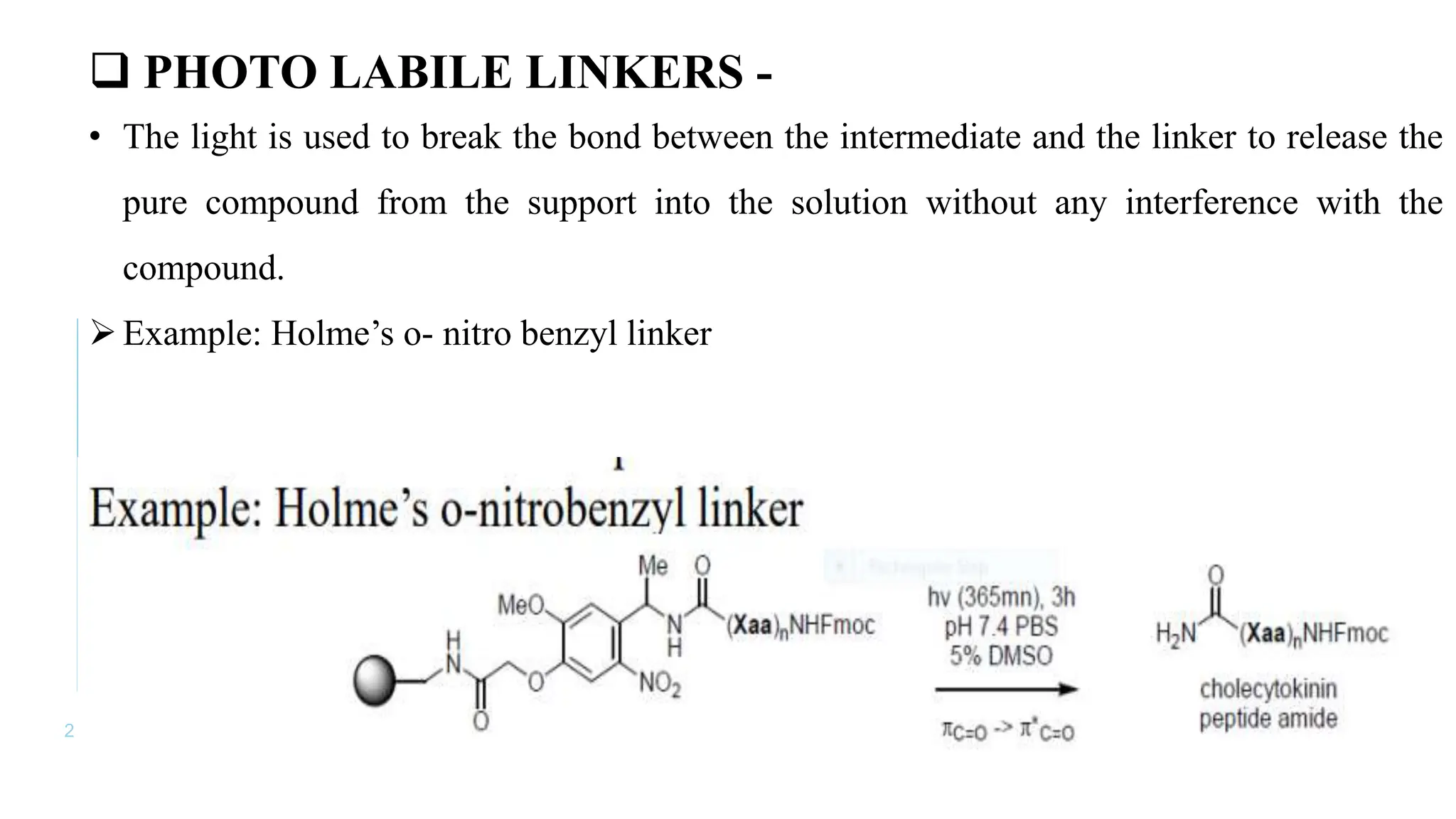
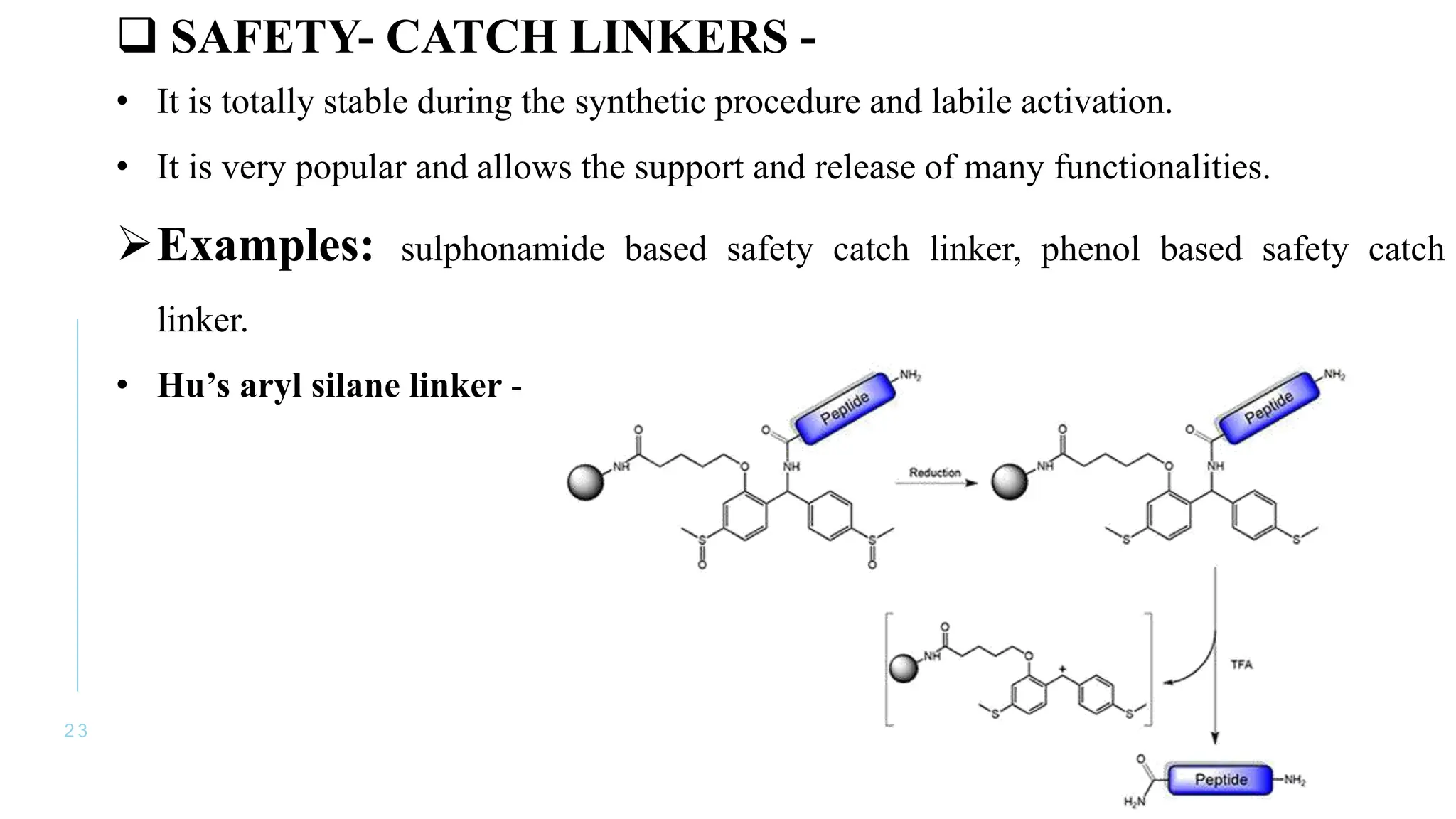
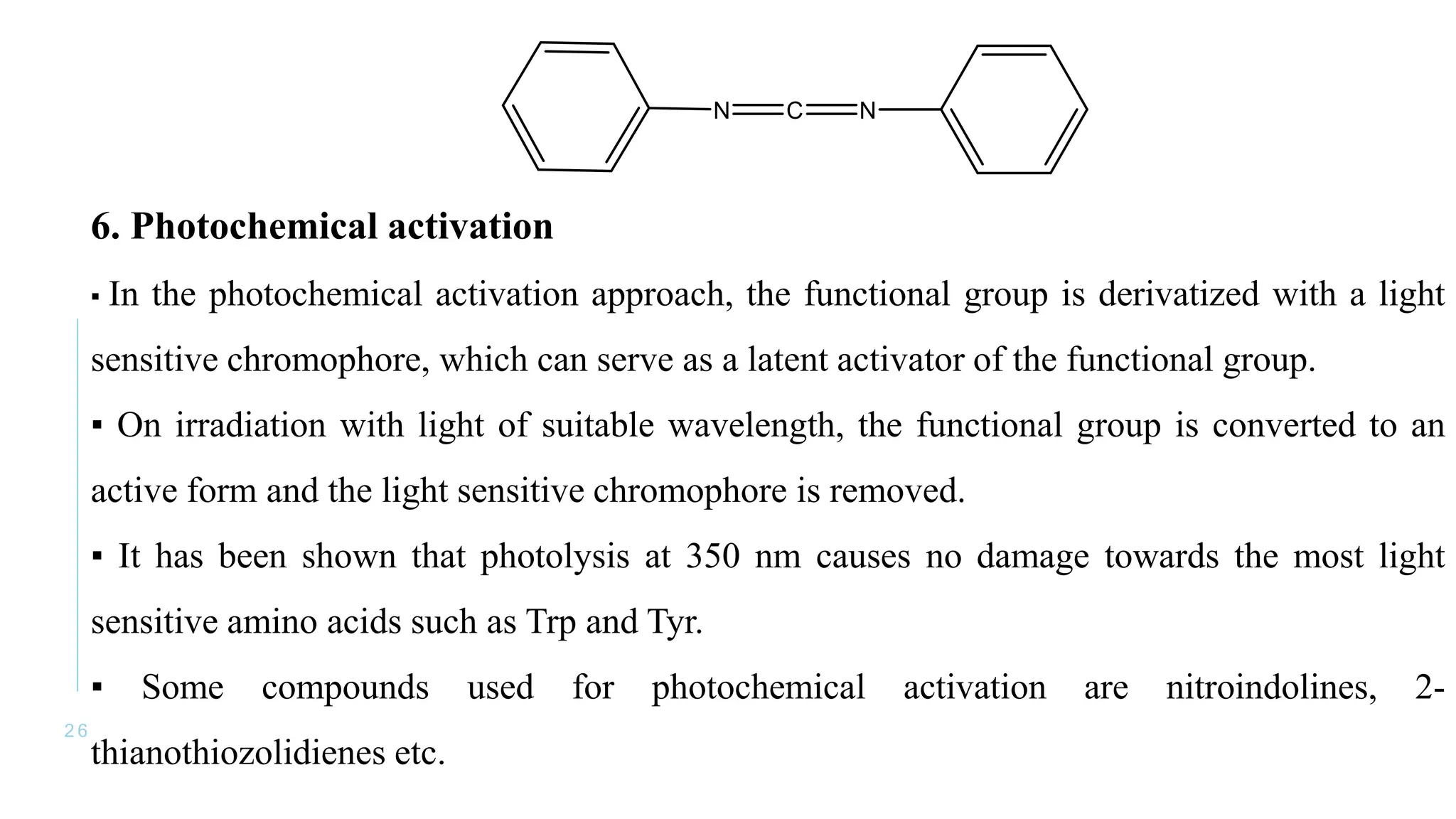
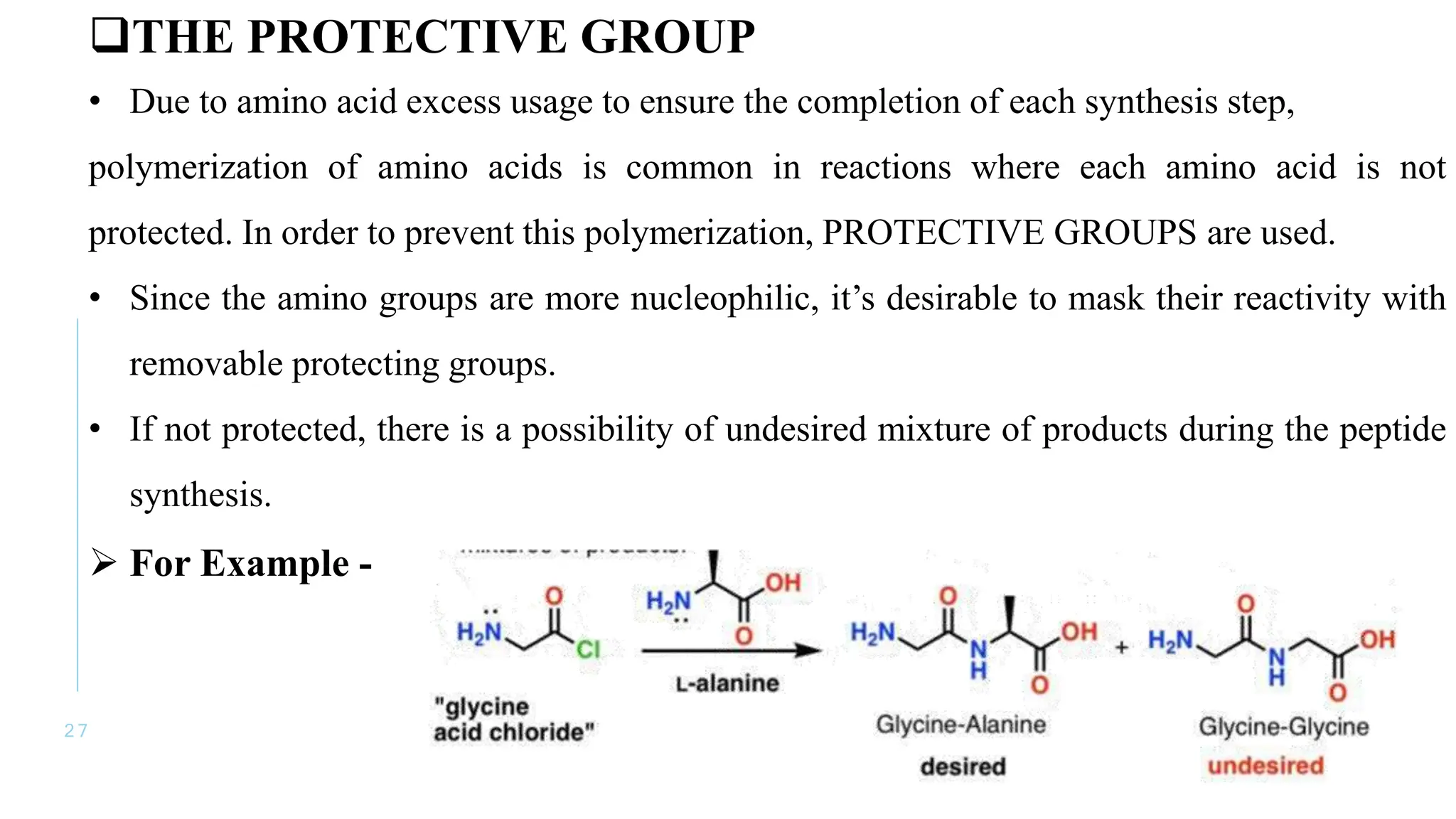
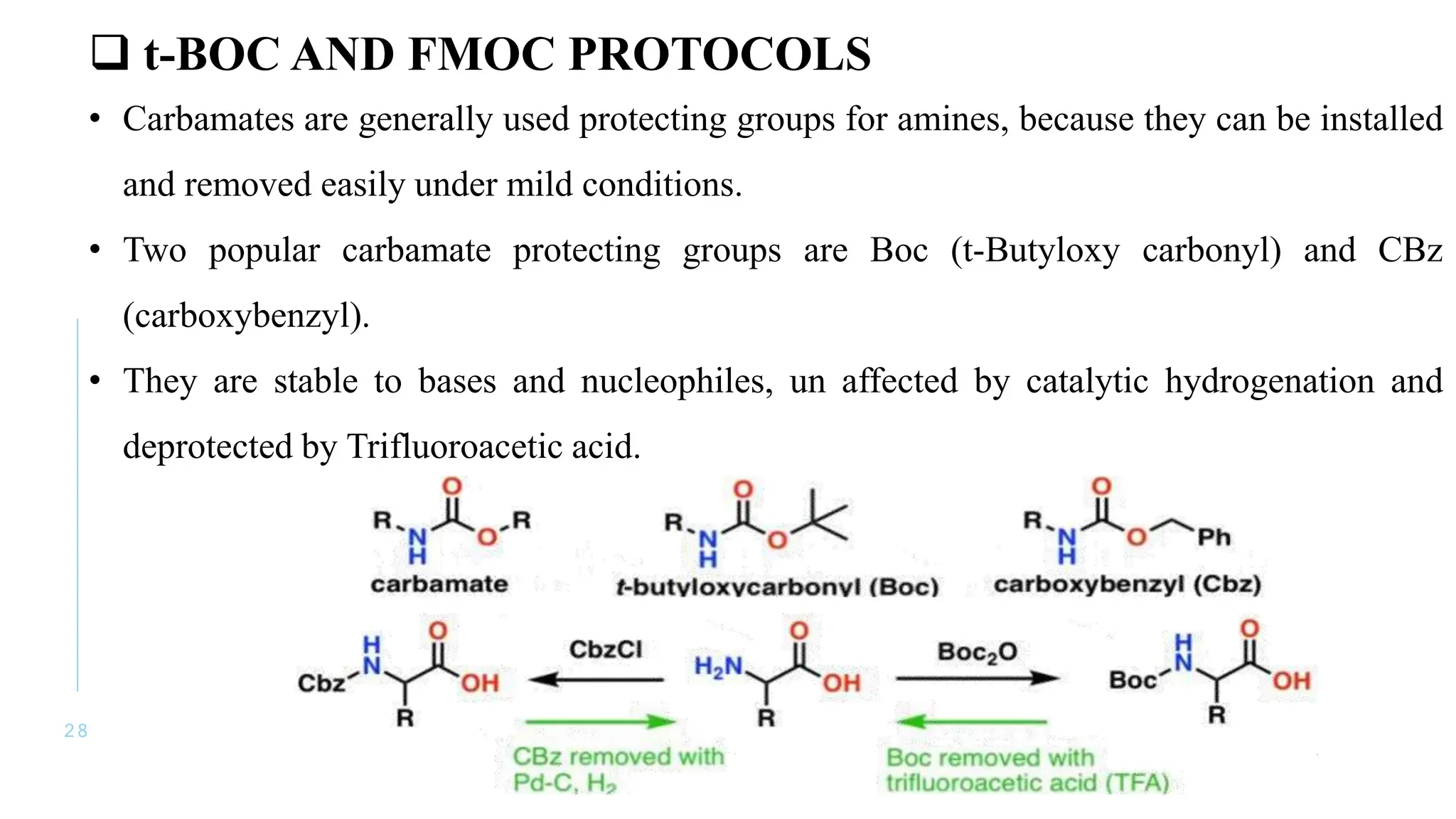
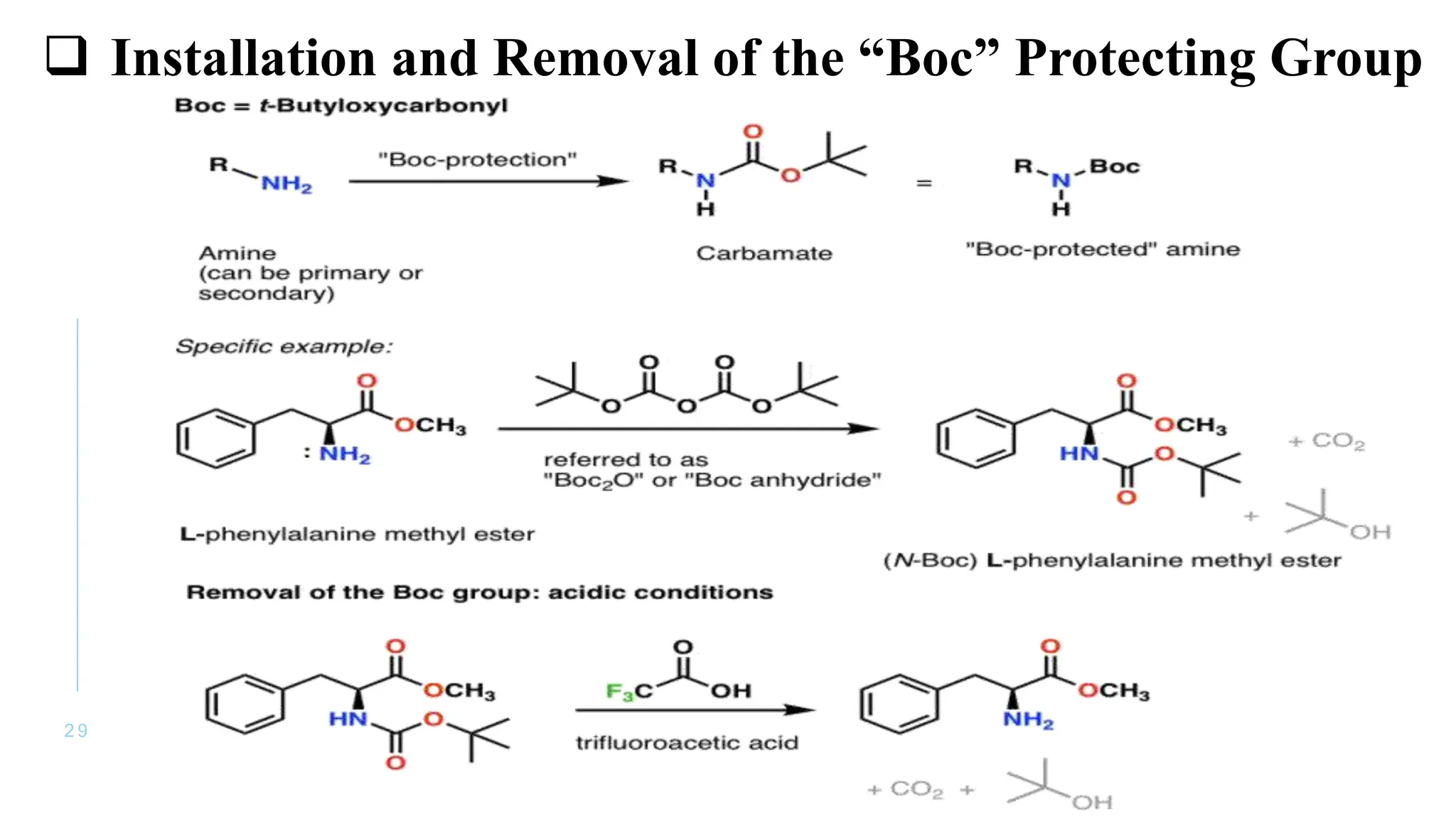
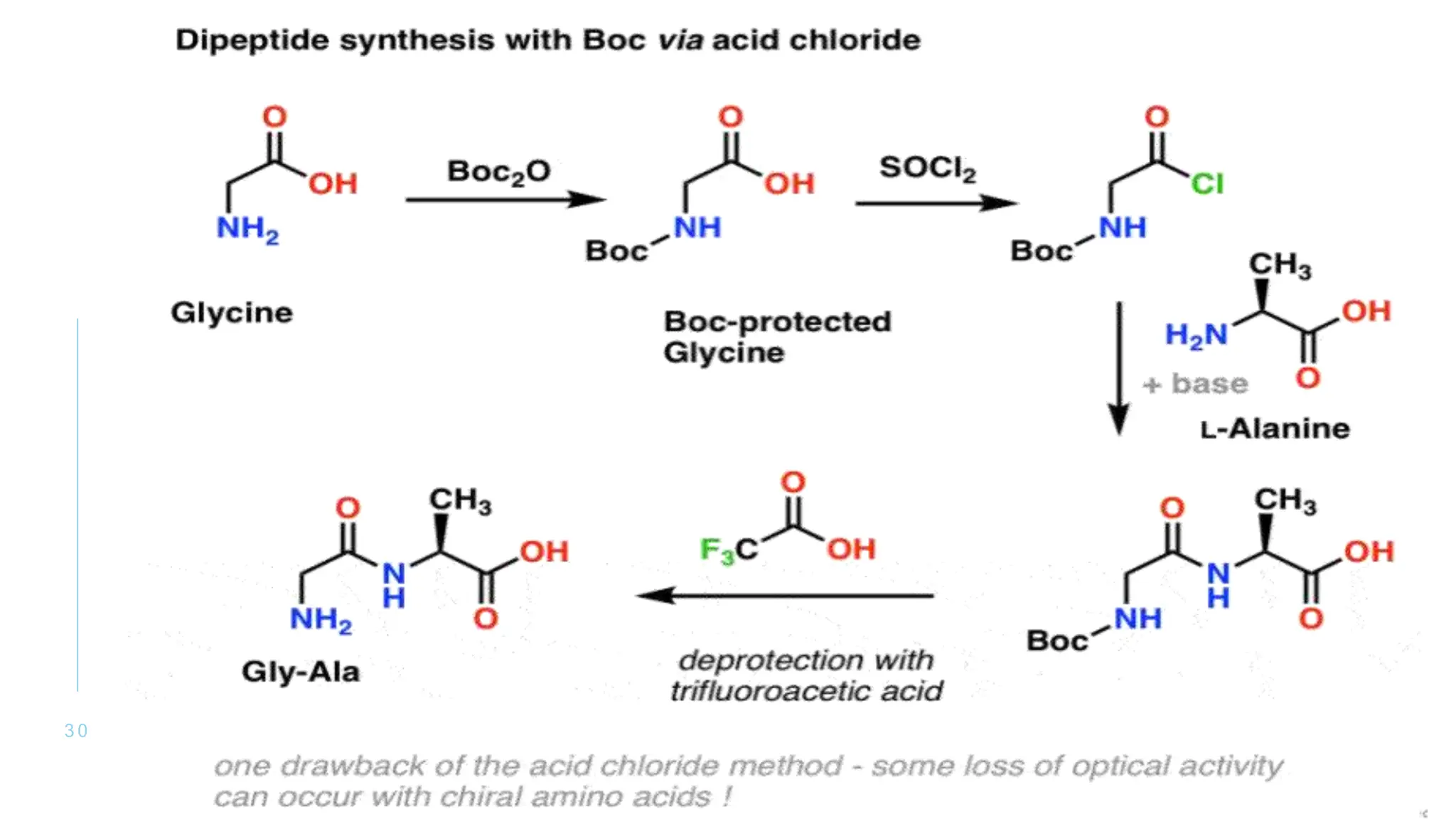
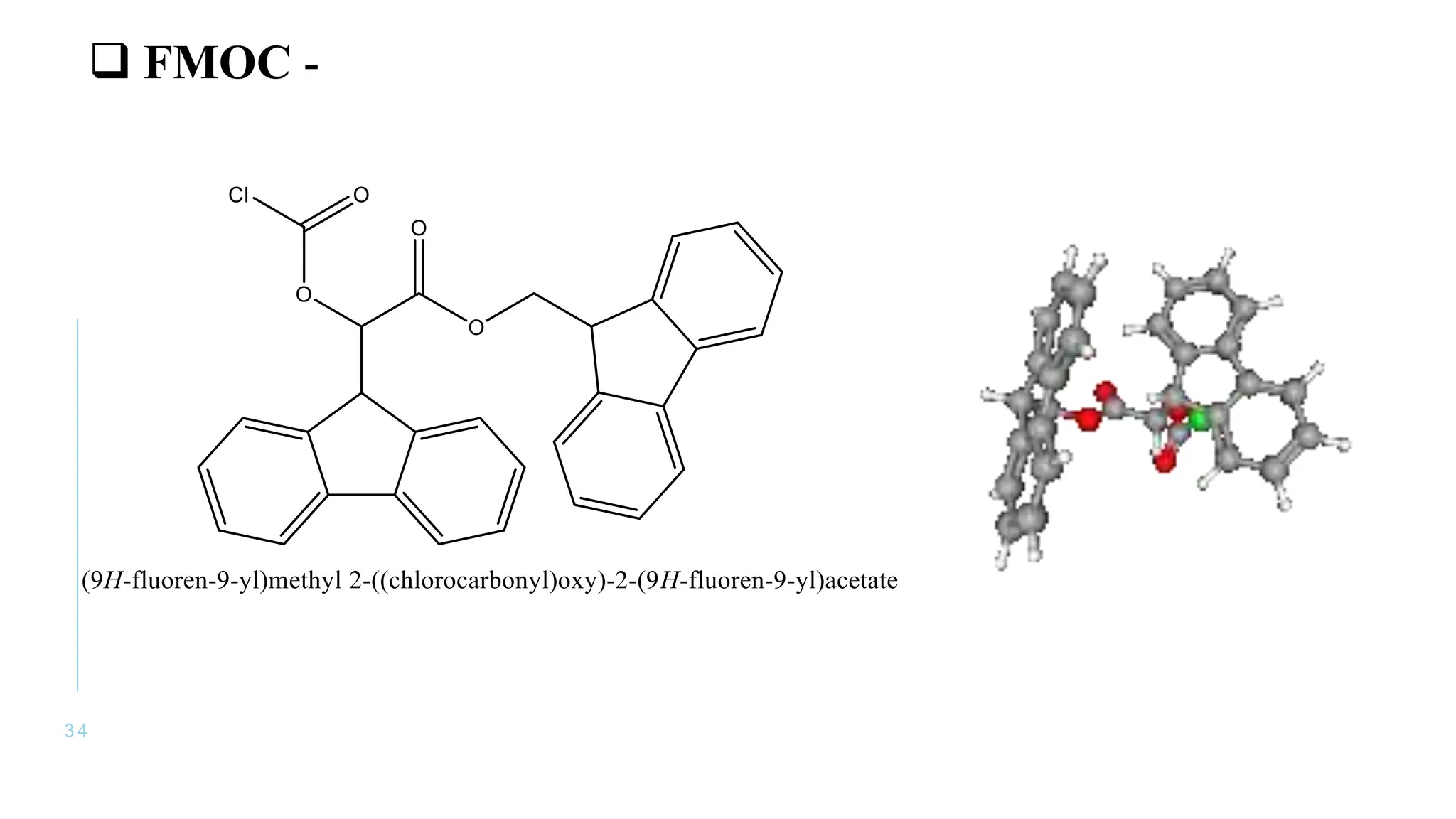
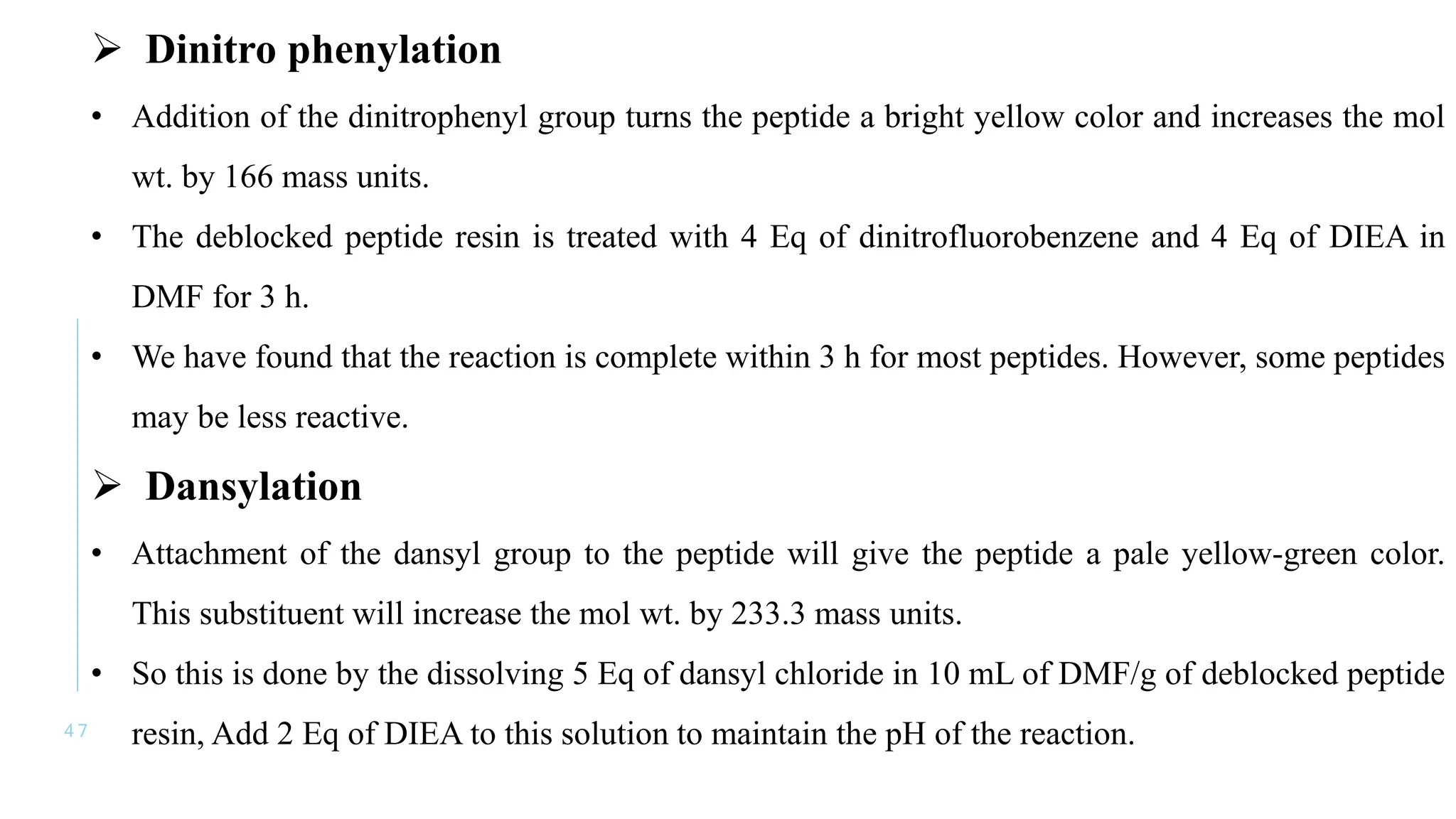
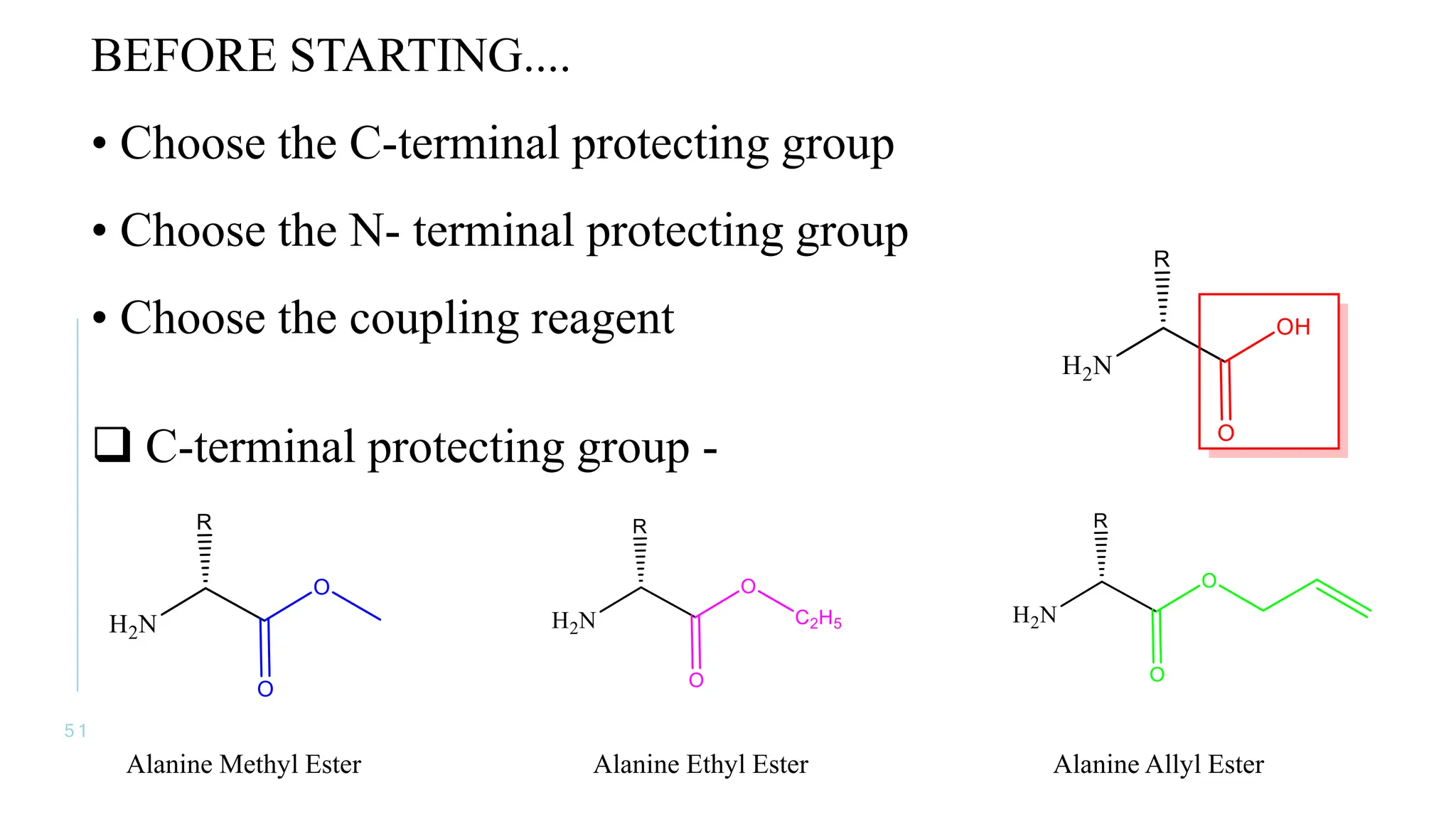
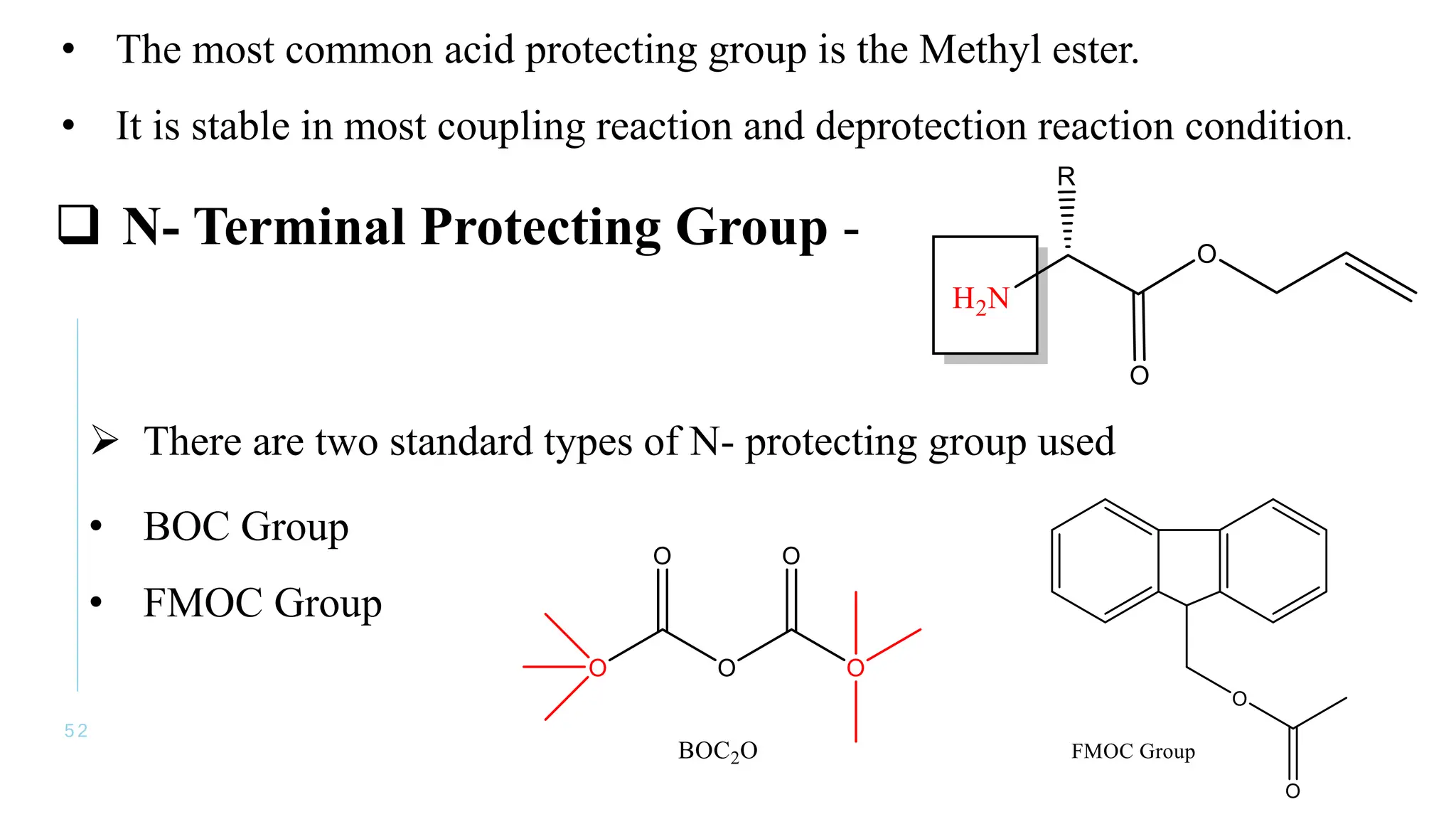
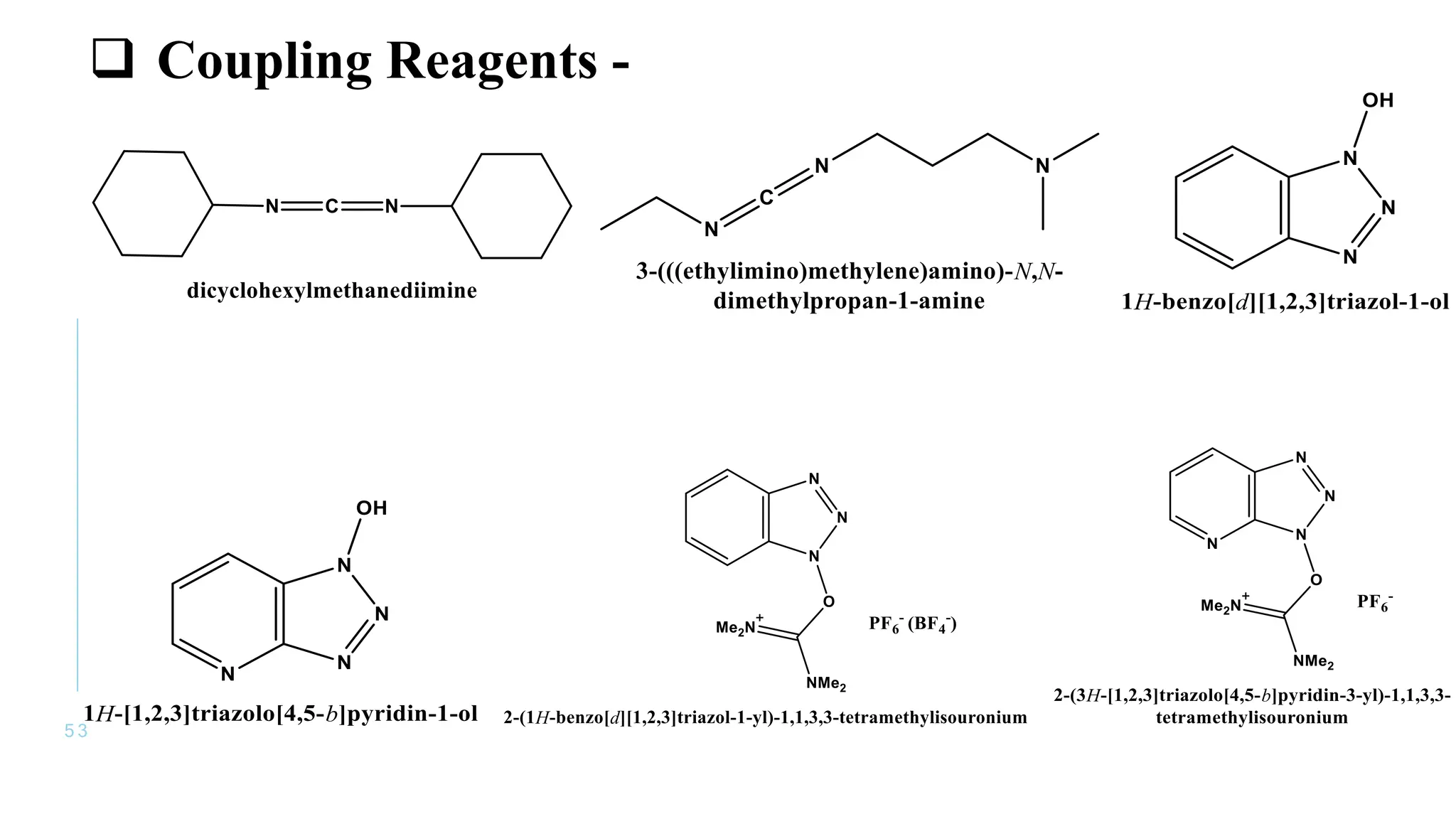
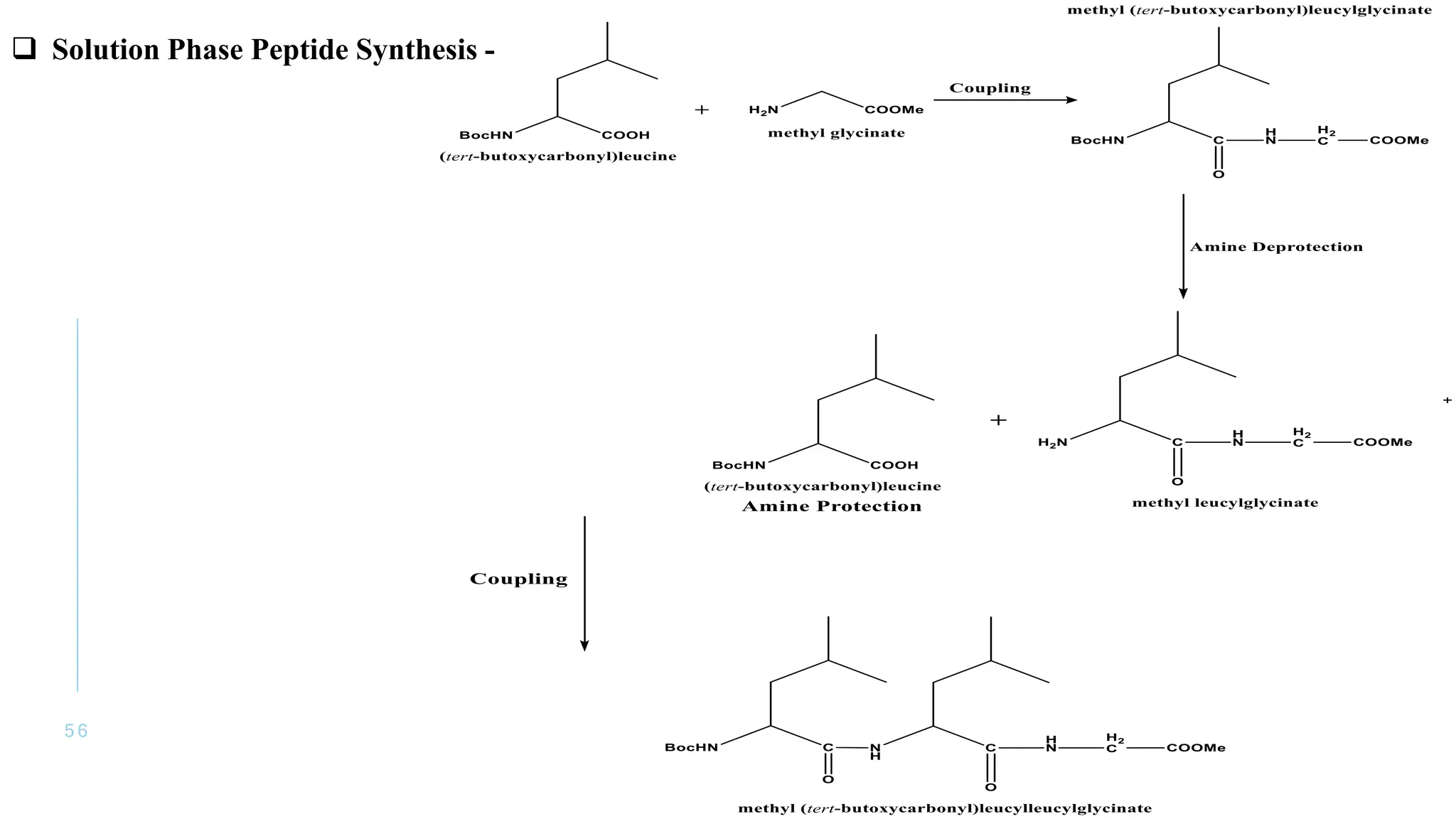
The document discusses solid phase peptide synthesis (SPPS) and solution phase peptide synthesis. It describes the key principles and steps of SPPS, including using a solid support resin, linkers to attach amino acids, protective groups, and repeating cycles of deprotection, wash, coupling, and wash. The two main SPPS methods discussed are Boc and Fmoc protocols, which differ in the protective groups and conditions used for deprotection and cleavage from the resin.