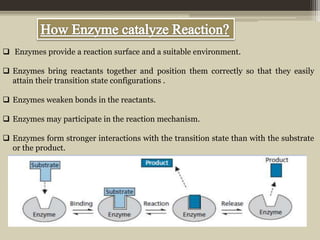
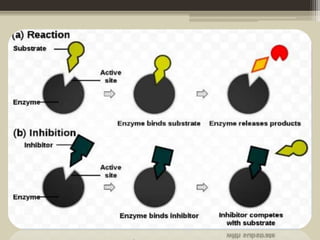
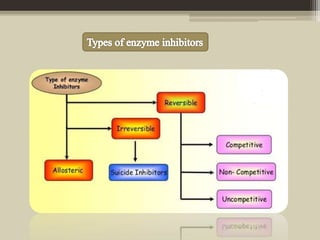
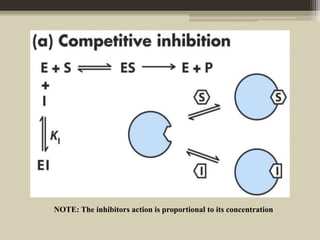
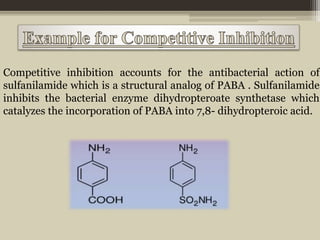
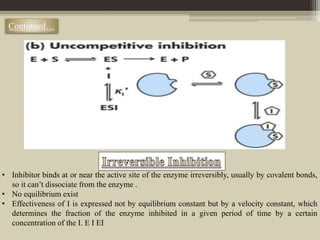
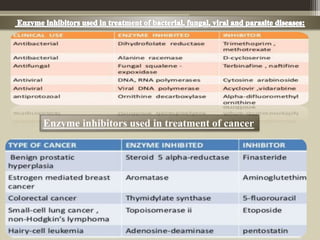
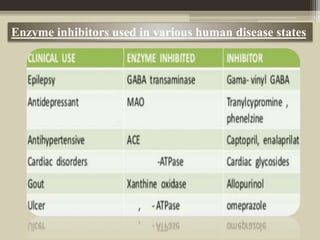
The document presents an overview of enzymes and enzyme inhibitors, detailing their classification, mechanisms, and applications in medicine and research. Enzyme inhibitors can be reversible or irreversible, affecting biochemical reactions by blocking enzyme activity, and they play significant roles in drug development for various diseases. Understanding the specificity and biochemical environment of these inhibitors is crucial for their therapeutic effectiveness.