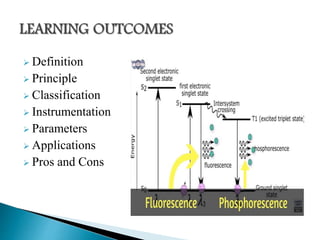
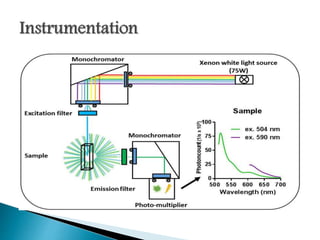
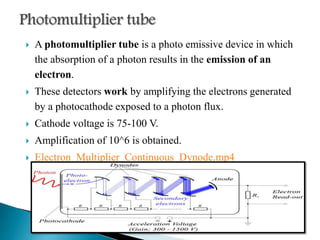
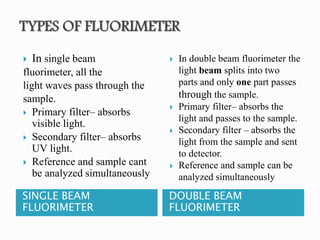
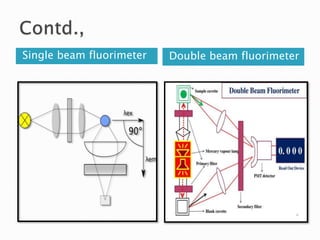
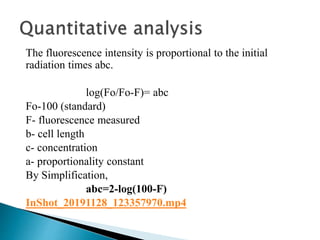
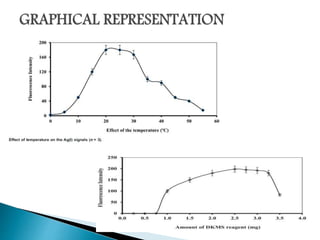
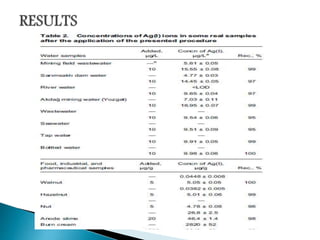
This document discusses the principles and applications of fluorimetry. It defines fluorescence as the emission of light from excited electrons returning to ground state. Fluorimetry involves using ultraviolet light to excite sample molecules, which then emit light of lower energy. Factors that influence fluorescence intensity are discussed. Common instrumentation includes light sources, monochromators, cuvettes, detectors, and data analyzers. Applications include determining inorganic substances, proteins, and steroids. Two case studies on analyzing glucose in milk and silver in food/water samples using fluorimetry are presented.