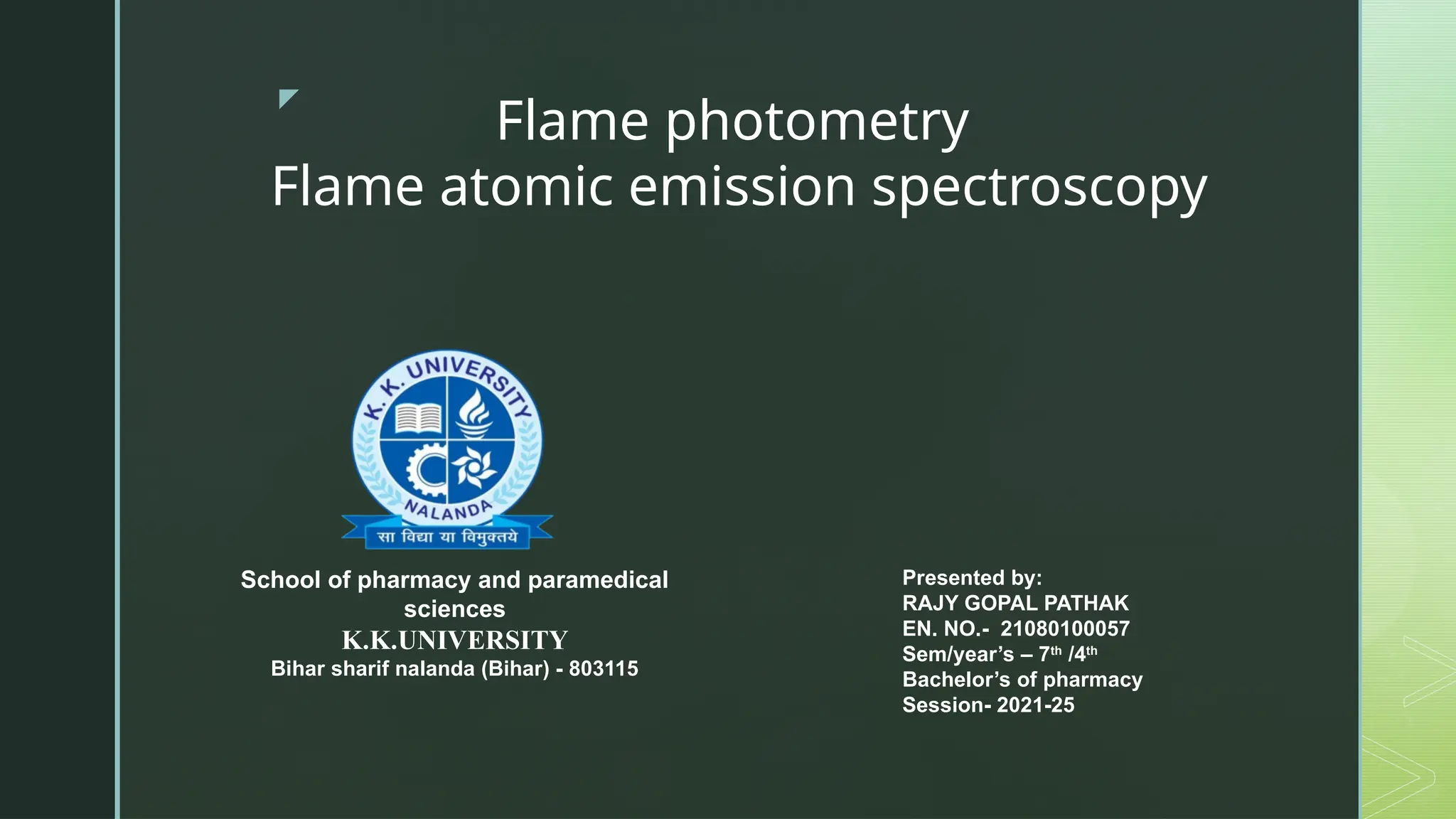
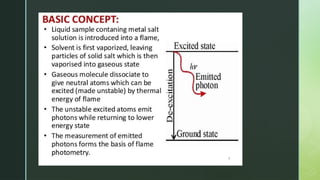
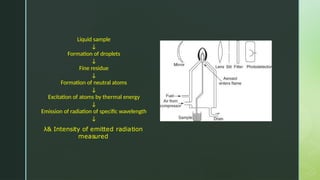
Flame photometry, also known as flame emission spectroscopy, is an analytical technique developed in the 1980s to measure low concentrations of alkali and alkaline earth metals in solutions by analyzing the emitted light intensity when these metals are introduced into a flame. The process involves several steps, including desolvation, vaporization, atomization, excitation, and emission, with a photo detector used to measure the emitted radiation. While the method offers advantages like simplicity and sensitivity for detecting metals, it has limitations including inability to accurately measure inert gases and certain metals, as well as requirement for liquid samples.