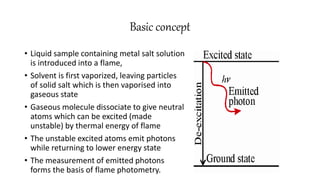
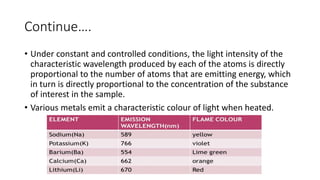
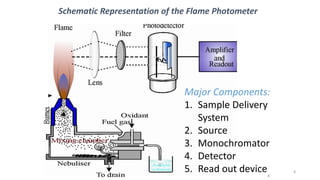
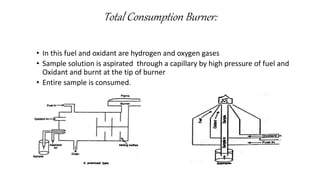
Flame photometry is a technique that uses the characteristic wavelengths of light emitted from atoms excited in a flame to determine the concentration of certain metal ions in a sample. When a sample containing metal ions is introduced into a flame, the metal atoms are excited and emit light at wavelengths characteristic of that element. The intensity of light emitted at that wavelength indicates the concentration of the metal ion in the original sample. Flame photometry can be used to determine concentrations of ions such as sodium, potassium, lithium, calcium, and others.