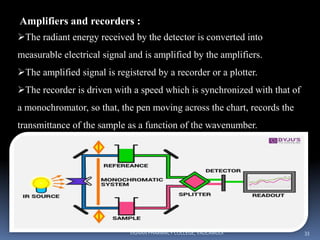
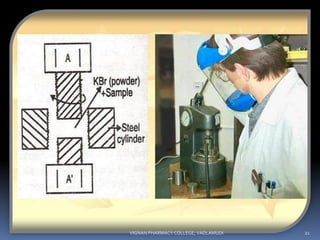
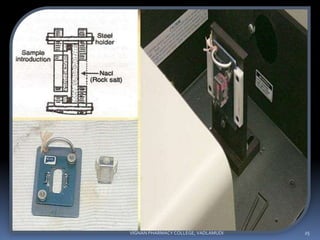
The document discusses various techniques for sample handling and preparation in infrared spectroscopy. It describes the different methods used for sampling solids, liquids, and gases. For solids, the key techniques discussed are running solids in solution, forming solid films, the mull technique, and pressed pellet preparation using KBr. For liquids, cells made of materials like NaCl, KBr, and ThBr are used. Gas samples utilize larger cells of the same materials to compensate for lower molecule numbers. Proper sample preparation is necessary to obtain infrared spectra without interference from cell materials.
























![Monochromator:
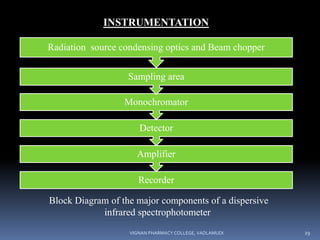
A monochromator is a means of separating wavelengths of the source
radiation.
The monochromator is used to separate polychromatic radiation into
a suitable monochromatic form [105].
This is achieved by means of prisms or diffraction grating.
A monochromator thus carries out three functions:
1 • It disperses the radiation according to its wave number
components.
2
• It restricts the radiation falling on the detector into a narrow wave
number range.
3
• It maintains the energy incident on the detector to an
approximately constant level.
VIGNAN PHARMACY COLLEGE, VADLAMUDI 31](https://image.slidesharecdn.com/pandu143-191110072119/85/Sampling-techniques-of-Infrared-Spectroscopy-31-320.jpg)