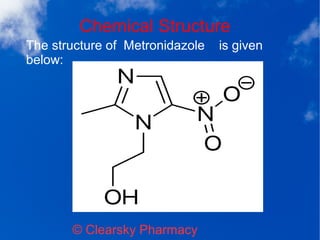
Metronidazole tablets, marketed as Flagyl, are used to treat various bacterial infections, including those affecting the vagina, stomach, skin, and respiratory tract, and are effective against anaerobic bacteria. The medication has specific dosage recommendations for conditions such as trichomoniasis and amebiasis, and it is important to avoid use during the first trimester of pregnancy due to potential risks. Side effects may include nausea, dizziness, and, in rare cases, serious neurological issues, and the use of alcohol should be avoided during treatment due to possible adverse reactions.