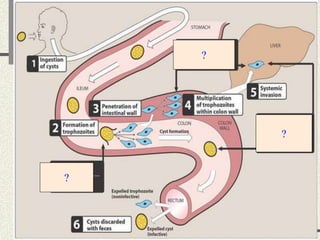
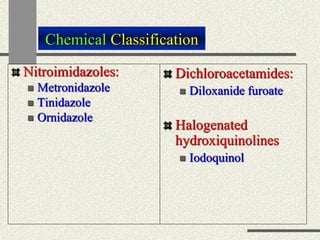
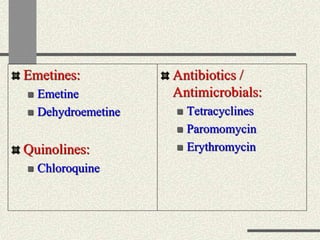
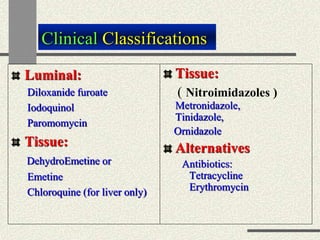
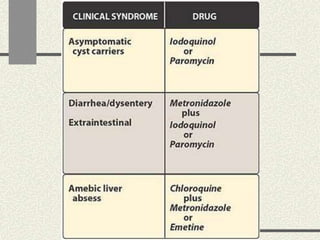
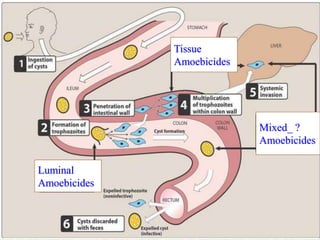
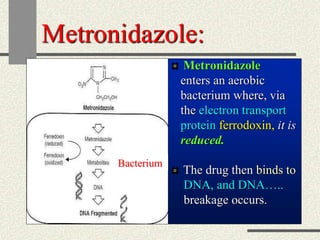
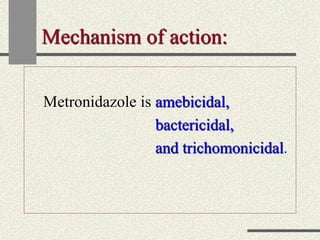
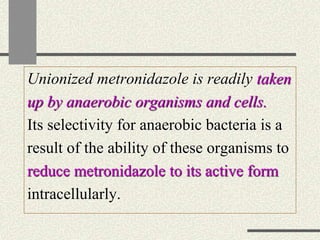
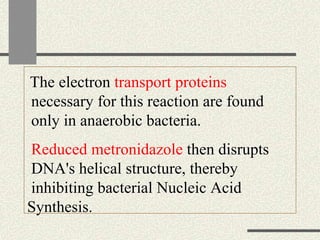
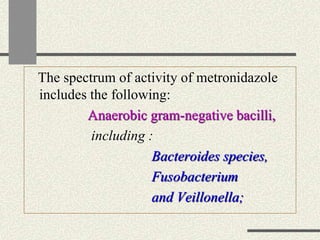
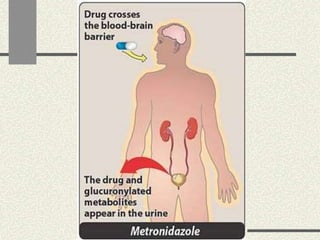
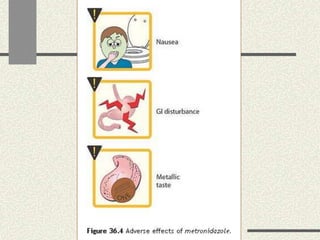
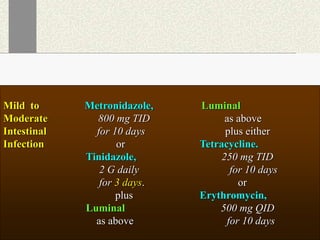
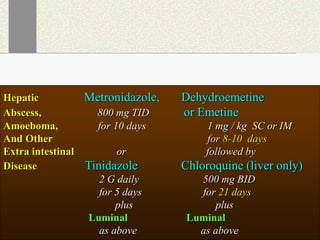
Amebiasis can be caused by two species of Entamoeba, with E. histolytica causing disease in 10% of cases. It may lead to colitis or liver abscesses. Metronidazole is usually the first-line treatment for intestinal and liver infections as it is effective against the trophozoite form. It is an antibiotic that works by disrupting the DNA of anaerobic bacteria and parasites. Other drugs used include diloxanide furoate or iodoquinol as luminal amebicides, and emetine or dehydroemetine for tissue infections.