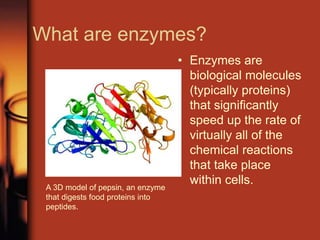
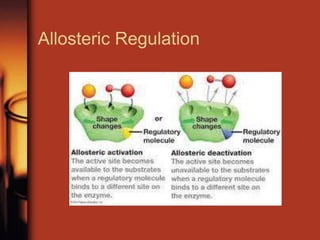
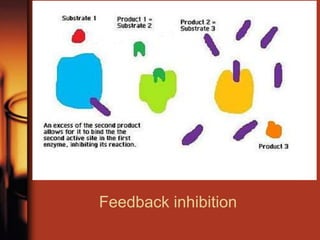
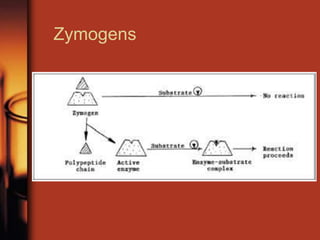
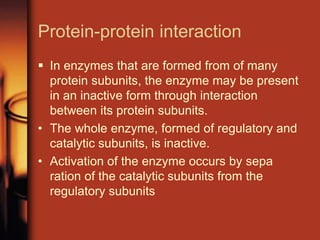
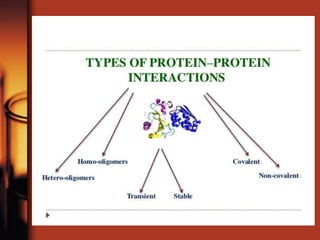
Enzymes are biological molecules, primarily proteins, that catalyze chemical reactions within cells, playing crucial roles in metabolism, digestion, and disease management. They are classified based on their function and how they facilitate reactions, while their activity is regulated through mechanisms like synthesis control and allosteric regulation. Enzymes also have industrial applications in food processing and chemical production, and their regulation is essential for maintaining homeostasis in organisms.