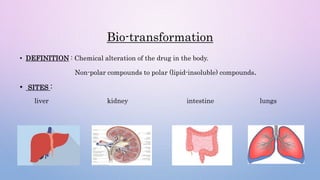
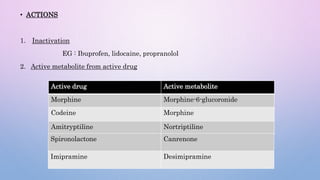
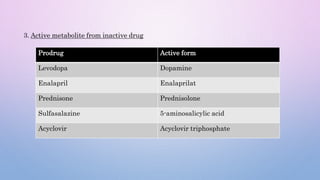
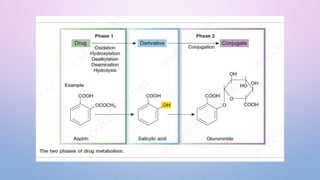
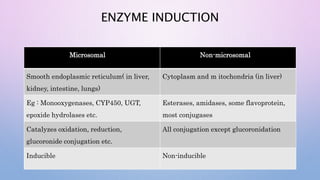
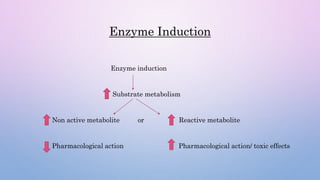
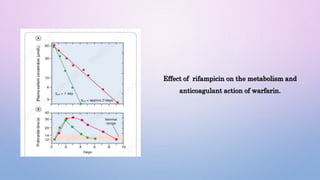
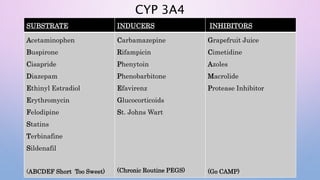
Microsomal enzymes like cytochrome P450 and UDP glucoronosyl transferases are important for drug metabolism in the liver and other tissues. Cytochrome P450 enzymes catalyze oxidation, reduction, and other phase I reactions. UDP glucoronosyl transferases catalyze phase II conjugation reactions like glucoronidation. Drug metabolism can be induced or inhibited by other drugs and environmental factors, leading to potential drug-drug interactions. A better understanding of an individual's genetic polymorphisms and environmental factors can help optimize drug therapy and avoid adverse reactions.