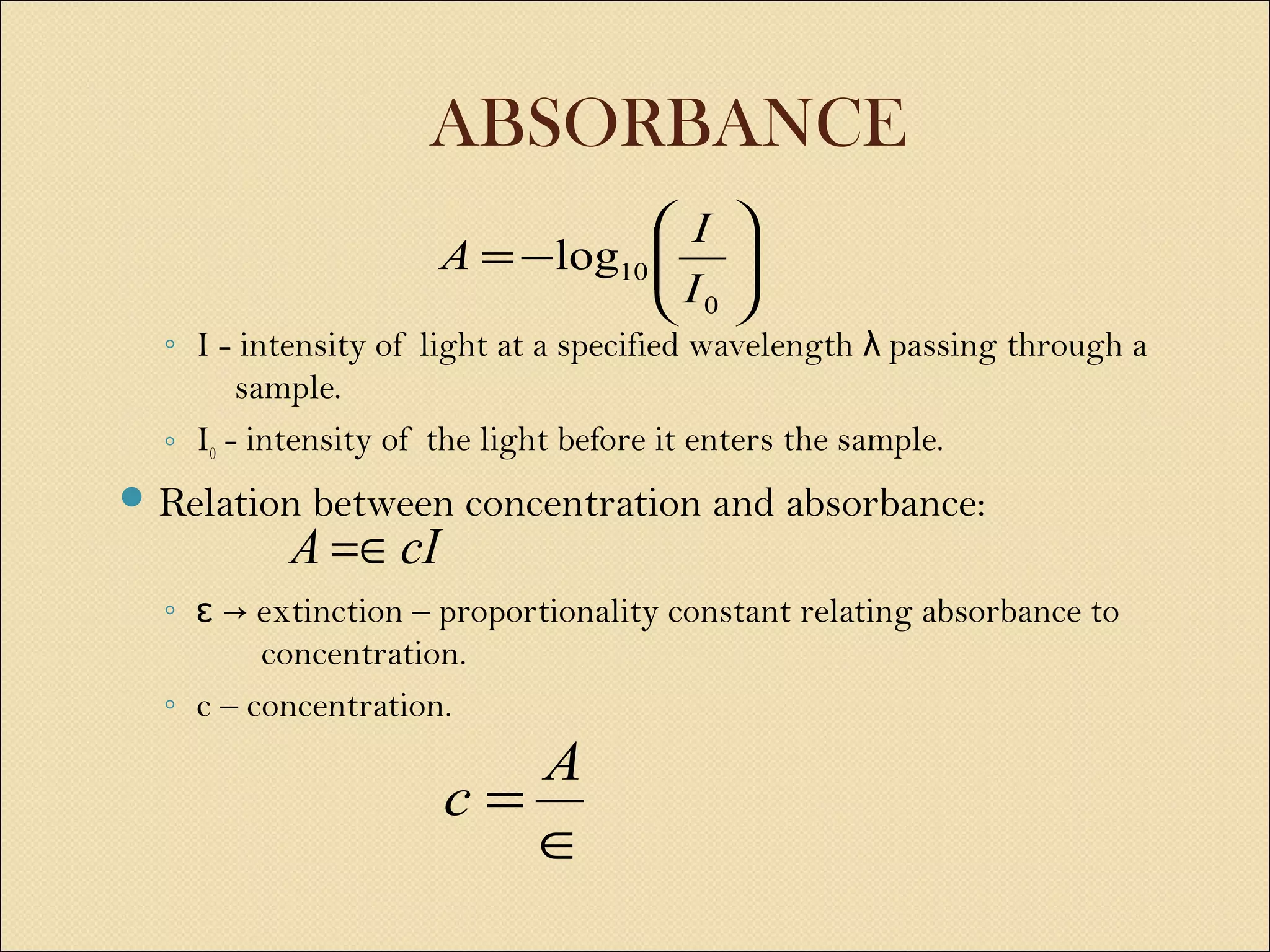
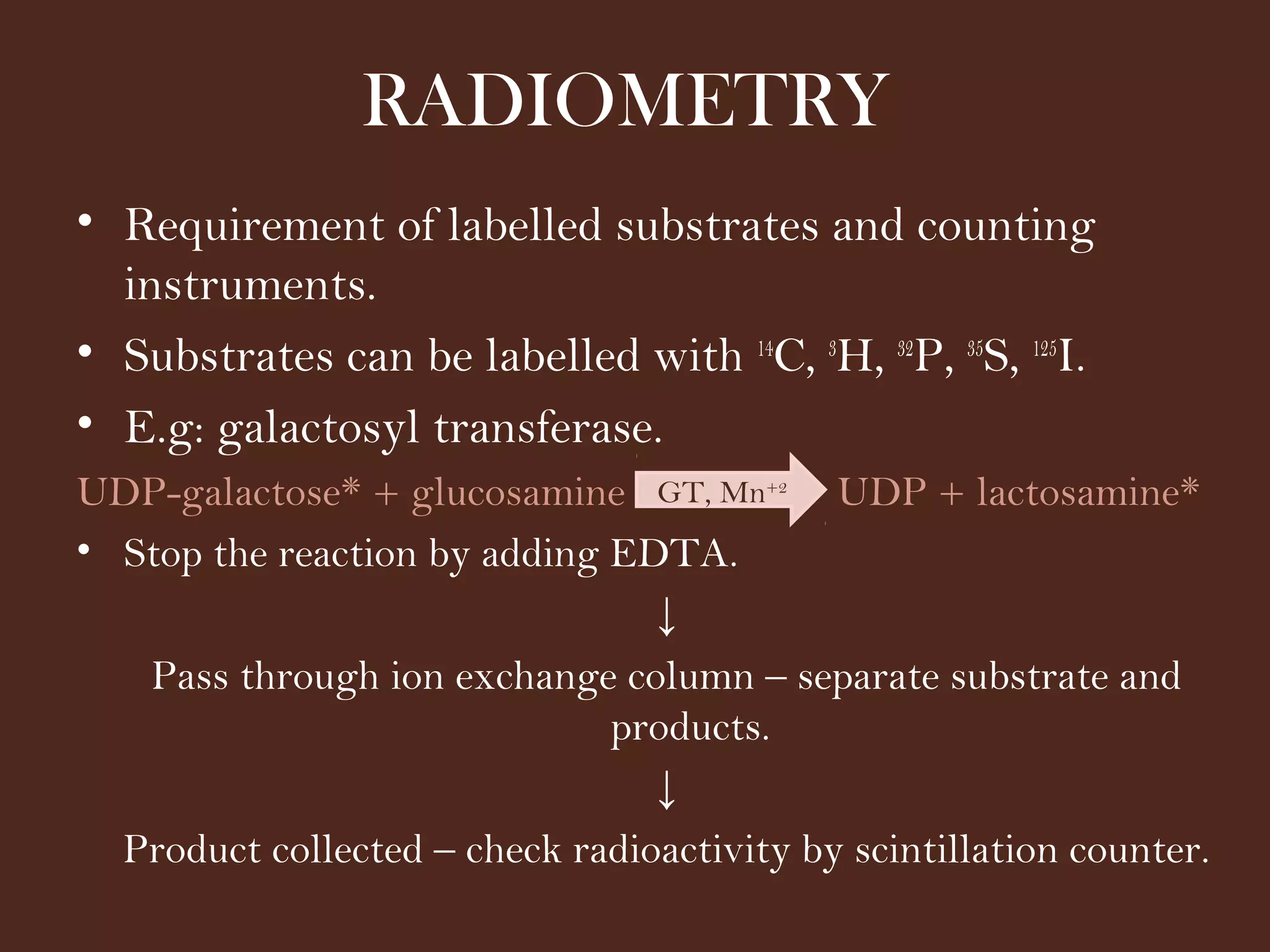
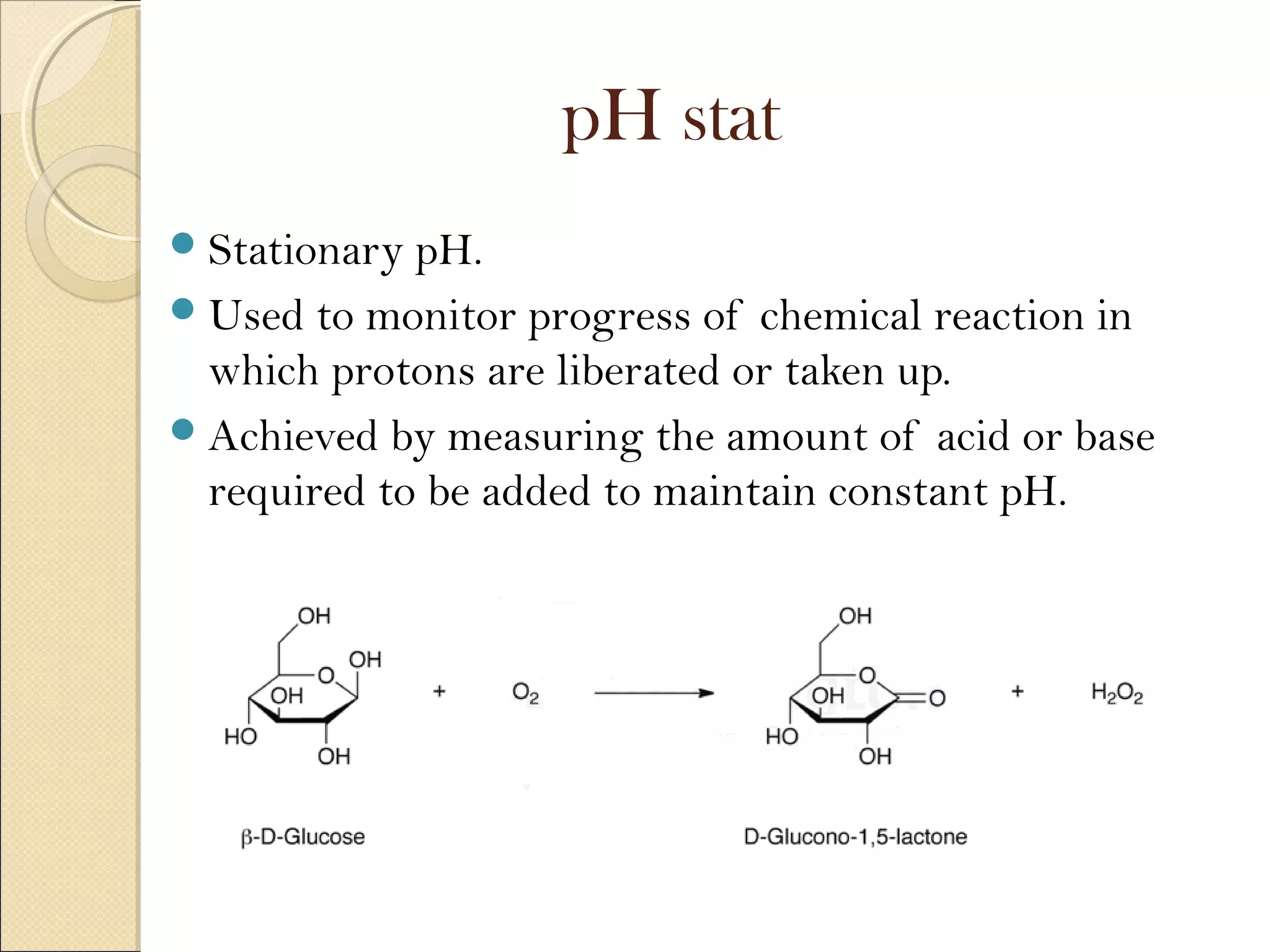
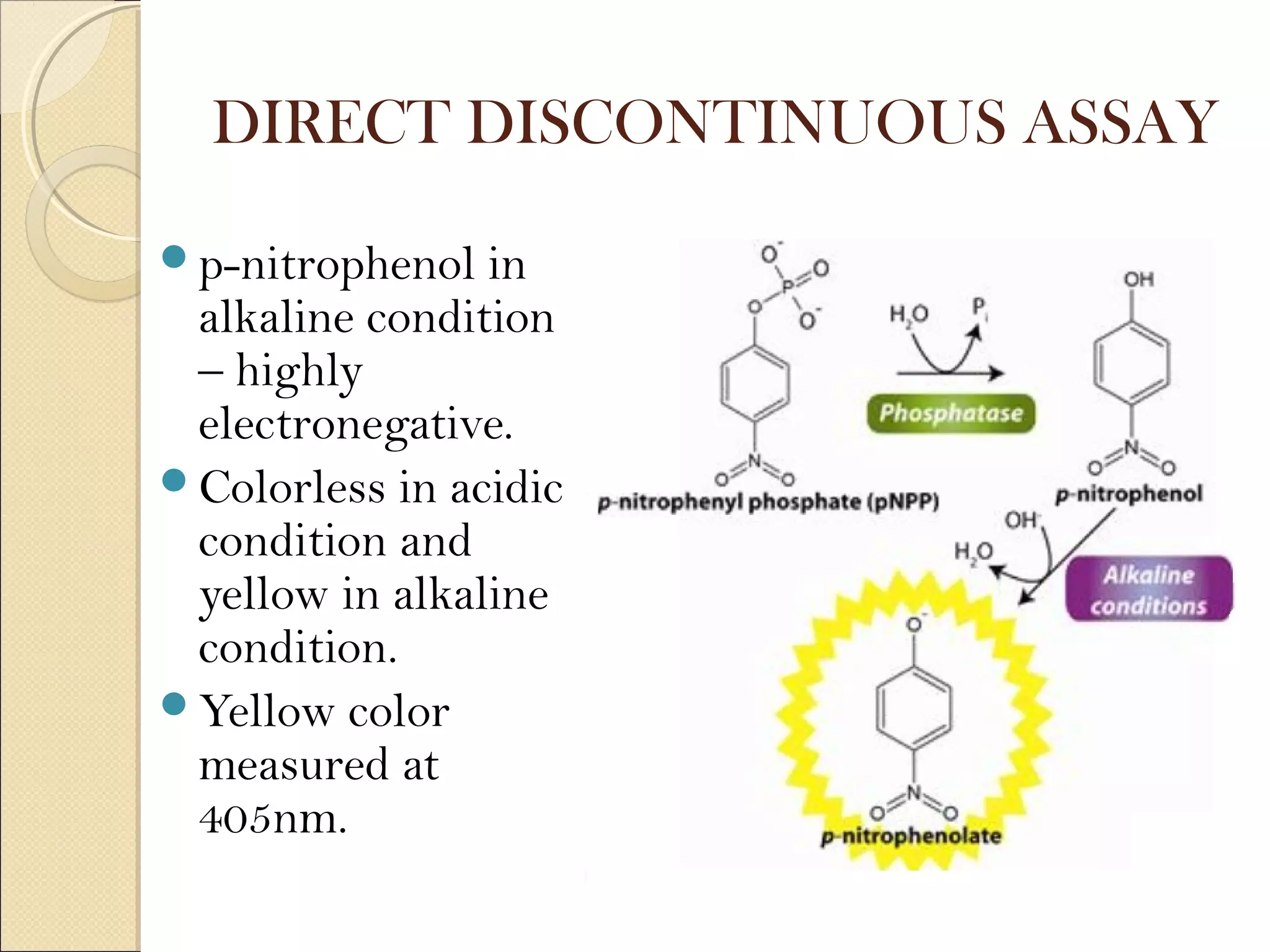
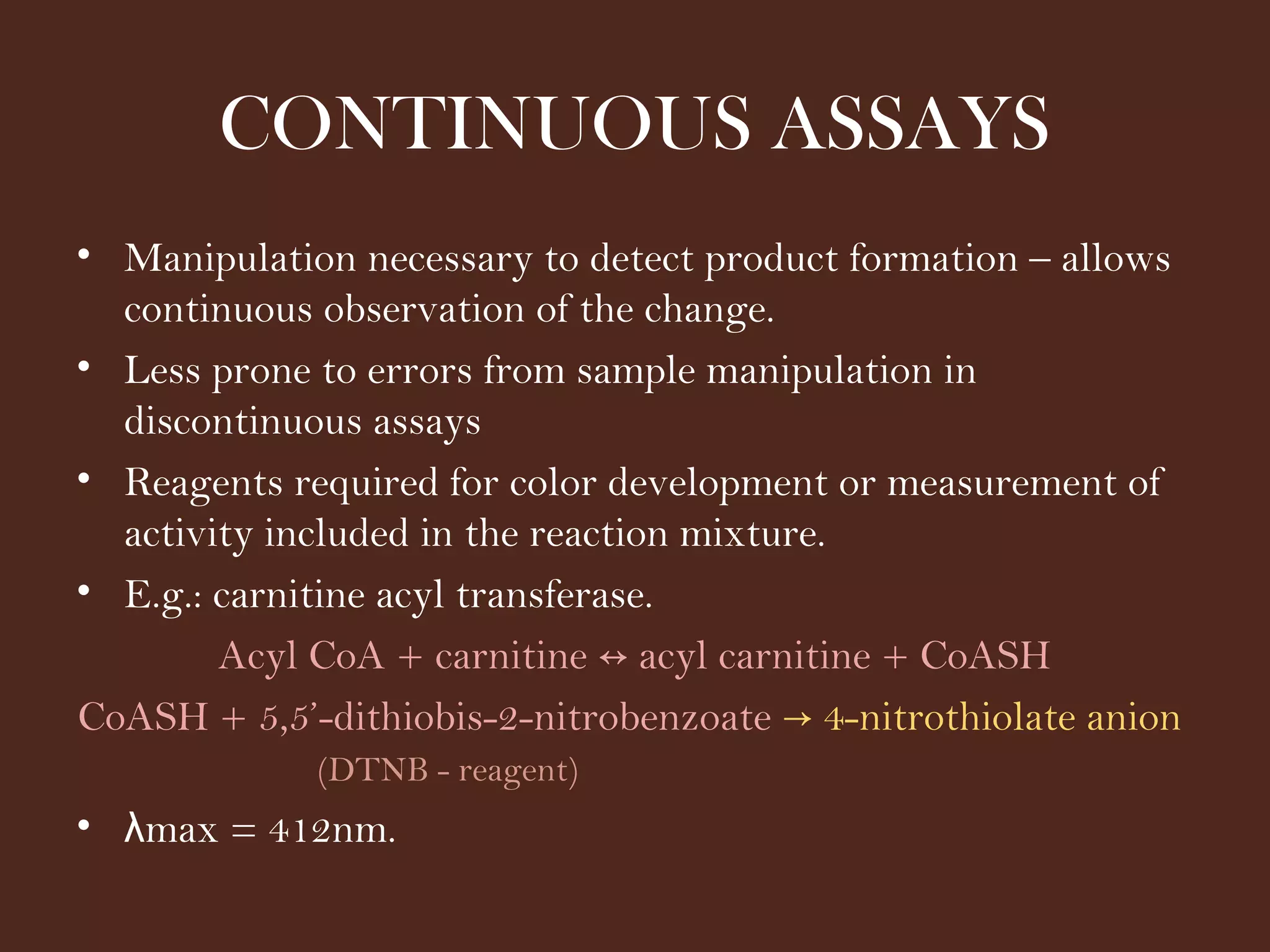
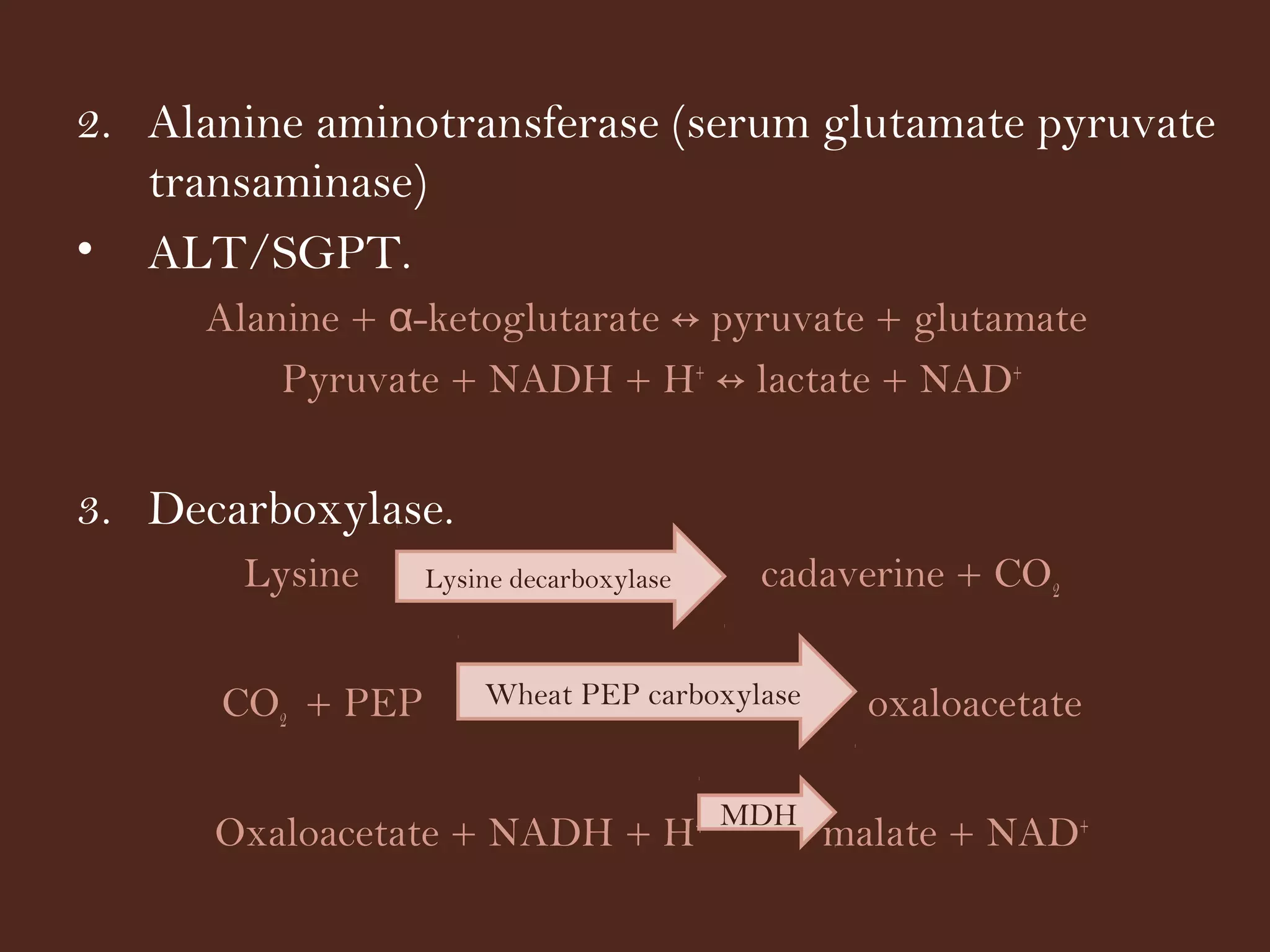
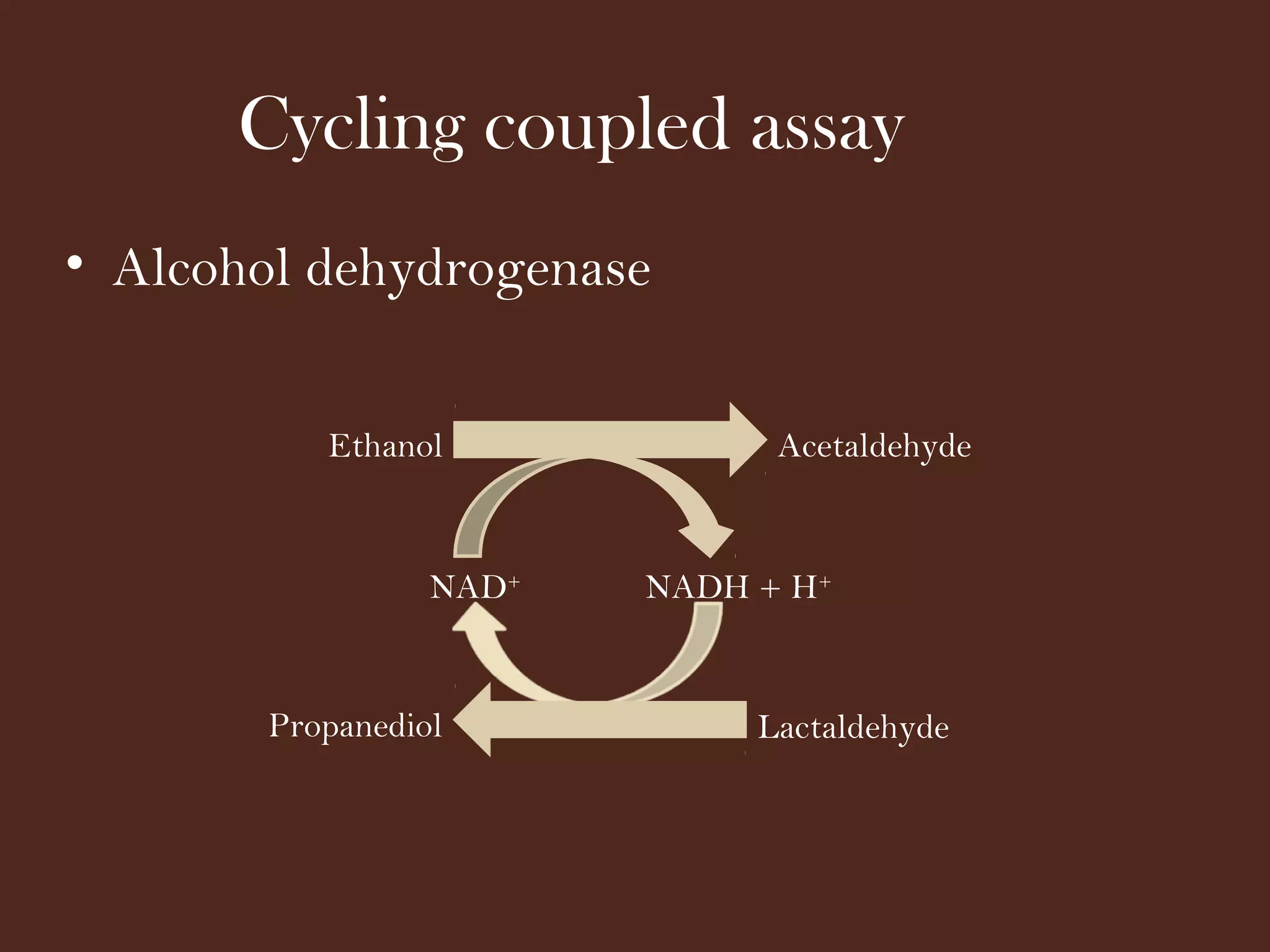
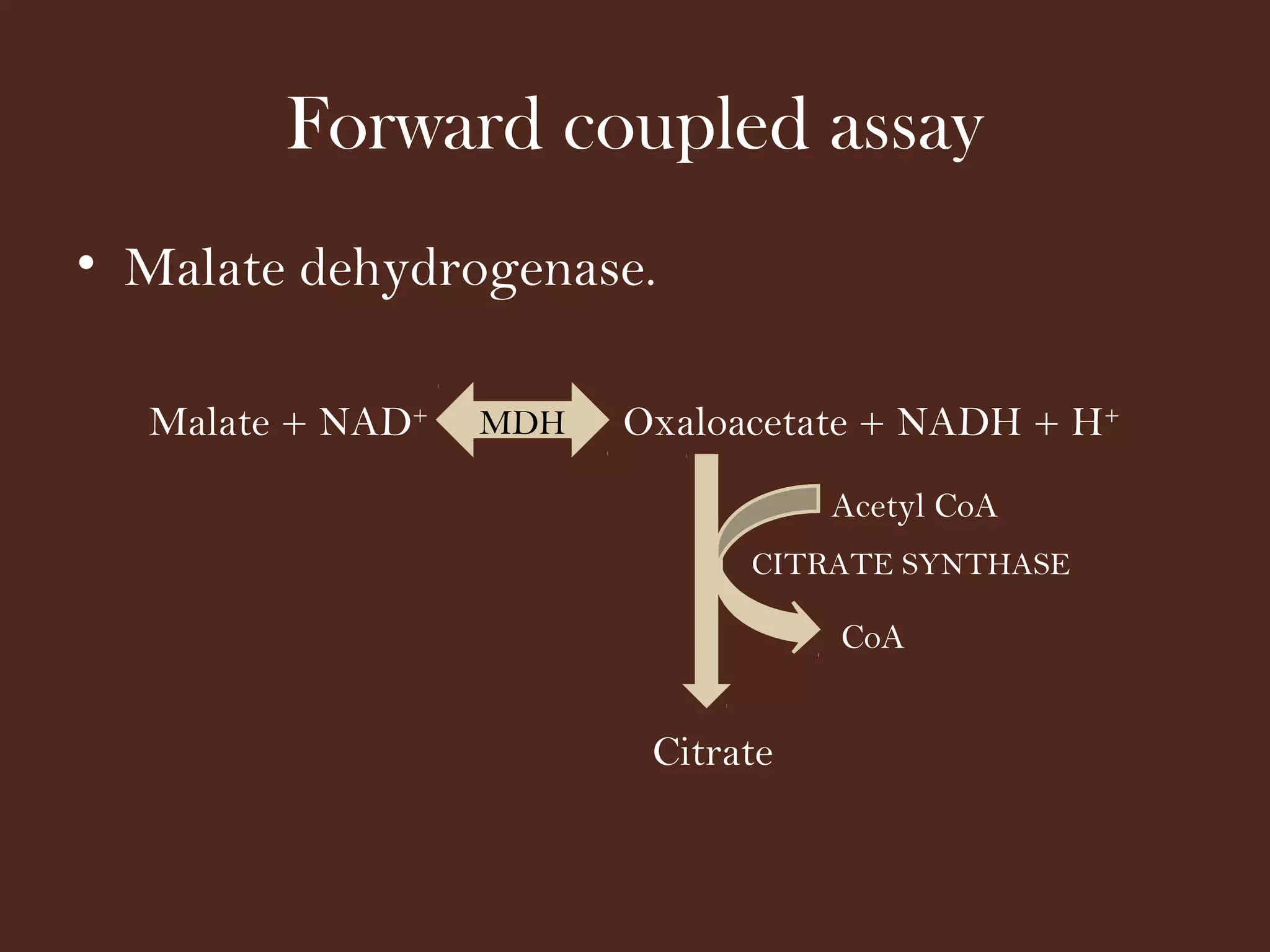
The document discusses various methods for measuring enzyme activity through enzyme assays, highlighting direct continuous, discontinuous, and indirect assays. It elaborates on specific techniques like turbidimetry, fluorescence, radiometry, and coupled assays, including their sensitivity and methodologies. Additionally, it stresses the importance of parameters such as pH and temperature on assay validity and reaction rates.