The document discusses various methods for performing enzyme assays, including kinetic determination of catalytic activity, coupled kinetic assays, and radioimmunoassay. It notes that enzyme activity is measured by determining the rate of substrate conversion to products under specified conditions. For kinetic assays, the initial velocity is proportional to enzyme concentration when substrate levels are saturating. Coupled assays allow indirect measurement of a reaction by linking it to a second reaction that produces a detectable product. Radioimmunoassay uses competitive binding between unlabeled antigen in the sample and radioactively labeled antigen to antibodies to quantify antigen concentration. The document provides details on setting up reliable and reproducible assay systems.


![Assay By Kinetic Determination Of Catalytic
Activity
to find out how much substrate is capable of converting to product in a given
time under specified conditions.
the initial velocity of an enzyme-catalyzed reaction taking place under
conditions where the Briggs-Haldane steady-state assumptions are valid is given
by
v0 = k2[E0][S0]
([S0 ] + Km),
here k2 is the rate constant relating to product formation
(i.e. kca1).
If the value of [So] used in a particular assay is fixed, the only variables are v0
and[Eo],
so vo =constant x [E0].](https://image.slidesharecdn.com/enzymeassaymethods-170822142408-230901060618-44fb54cb/85/enzymeassaymethods-170822142408-pdf-3-320.jpg)
![ in practice it is more reliably valid when the substrate concentration is high enough to be
approximately saturating.
There are two main reasons for this.
1, integration of the appropriate rate equation shows that the linear, steady-state phase of the
reaction is more prolonged as the degree of enzyme saturation is increased (all other factors being
equal).
In simple terms, this is because the rate of utilization of substrate becomes less significant in relation
to the total concentration of substrate present as [So] is increased. For this reason more accurate
estimates of v0 can be obtained at high rather than at low [So] values
2, at high [So],
([S0] + Km) = [S0],
so v0 = k2[E0] and v0 does not vary with small changes in [S0].
This means that reproducible results can be obtained from an assay system without it being
necessary to fix the substrate concentration at an extremely precise value, provided [So] is sufficient to
almost saturate the enzyme. Hence the setting-up of a reliable assay system is more convenient the
higher the chosen value of [S0].](https://image.slidesharecdn.com/enzymeassaymethods-170822142408-230901060618-44fb54cb/85/enzymeassaymethods-170822142408-pdf-4-320.jpg)
![ According to the Michaelis-Menten equation, an enzyme is only completely
saturated by substrate at infinite substrate concentration, so it is necessary to talk in
terms of near-saturation rather than complete saturation.
the vo/Vmax ratio to be calculated for each initial substrate concentration, provided
the Km value is known.
It should be clearly understood that the vo/Vmax ratios and fractional saturation
values are independent of [E0], provided [S0] » [Eo], However, the actual values of
v0 and Vmax do vary with [Eo], which is the whole point of enzyme assay by kinetic
methods](https://image.slidesharecdn.com/enzymeassaymethods-170822142408-230901060618-44fb54cb/85/enzymeassaymethods-170822142408-pdf-5-320.jpg)




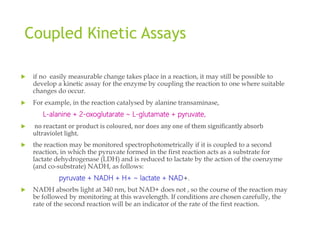
![ Let us consider the general situation:,
where E1 is the enzyme catalysing the primary reaction and E2 the enzyme catalysing the
indicator reaction.
At zero time, the concentrations of B and C will be zero, and the concentration of A
should be fixed and non-limiting .
If there are any second substrates or cofactors for E1 or E2 (e.g. NADH is a co
substrate in the example where E2 is LDH), then these should also be present initially
at fixed and non-limiting concentrations.
As the reaction proceeds, the concentration of B (pyruvate in the example where E2 is
LDH) will start to rise from its initial value of zero. In general, the rate of change of [B]
is given by
d[B]/dt = v1 - v2 ,
where v1 is the velocity of the primary reaction and v2 the velocity of the
indicator reaction. As with the velocity of any single-enzyme system, v1 should reach a
constant value almost instantaneously and remain at this value over the period of interest
(the next few minutes). In contrast, v2 will have an initial value of zero (since [B] is initially
zero) and will rise as [B] rises](https://image.slidesharecdn.com/enzymeassaymethods-170822142408-230901060618-44fb54cb/85/enzymeassaymethods-170822142408-pdf-11-320.jpg)
![ according to the Michaelis Menten equation
related to the reaction catalysed by the enzyme E2•
Therefore,
Integration of the expression for d[B]/dt shows that the time taken for this
steady-state to be established (or at least very nearly established) is
directly proportional to Km B Vmax B. Now, Kmax is a constant
characteristic of the enzyme E2, but Vmax is directly proportional to the
concentration of E2](https://image.slidesharecdn.com/enzymeassaymethods-170822142408-230901060618-44fb54cb/85/enzymeassaymethods-170822142408-pdf-12-320.jpg)
![ Hence, the larger the concentration of E2, the quicker is the
establishment of a near steady-state for the overall system, with v1 =
v2•
If there is so little E2 present that Vmax is less than vi. then a steady-
state can never be set up, [B] will continue to rise and v2 can never
reach the value of v1.
It is therefore important that sufficient of the indicator enzyme is
added to a coupled assay system to ensure that there is a minimum of
delay before a steady-state is established, and that the rate of the
indicator reaction at steady-state is a reasonable approximation to the
rate of the primary reaction.
As with a single-enzyme system, the validity of a coupled assay
procedure can be checked by assaying different amounts of the same
enzyme preparation: the specific activity should be found to be the
same in each case.](https://image.slidesharecdn.com/enzymeassaymethods-170822142408-230901060618-44fb54cb/85/enzymeassaymethods-170822142408-pdf-13-320.jpg)
![Radioimmuno assay [ R I A ]
The sample to be assayed is mixed with antibody and with a small amount of
the labelled antigen. The following reactions take place, and are allowed to
come to equilibrium
The antibody, with its bound antigen, is then separated from the free antigen and the
distribution of radioactive isotope between the free and bound antigen fractions is
investigated. It will be realized that the labelled antigen competes with the antigen in the
sample for the available binding sites on the antibody, so the higher the concentration of
antigen in the sample, the less radioactive antigen will be able to bind to the antibody and the
greater will be the radioactive content of the free antigen fraction. In this way, the
concentration of antigen in the sample can be estimated.
Antibodies for the RIA of enzymes may be prepared in the form of antisera by
immunizing rabbits with the required enzyme. For example, the blood of a rabbit
immunized in the footpad with human pancreatic a-amylase contains sufficient
antibodies within a few weeks to be usable as an antiserum.](https://image.slidesharecdn.com/enzymeassaymethods-170822142408-230901060618-44fb54cb/85/enzymeassaymethods-170822142408-pdf-14-320.jpg)


